The preprints of the #SOLIDARITY trial are out on MedRxiv. While many may lament that all four drugs tested did not show benefit, this is a remarkable trial for many reasons. Thread
First, this was another #MultiArmMultiStage #MAMS study design; @MRCCTU made this highly efficient trial design globally acclaimed with #STAMPEDE. The more recent #RECOVERY trial was another example of this.
@MaxParmarMRCUCL @Prof_Nick_James @PeterHorby @MartinLandray
@MaxParmarMRCUCL @Prof_Nick_James @PeterHorby @MartinLandray
What the #MAMS design does is enable testing multiple drugs simultaneously, flexibility to drop unpromising ones & add new promising ones even midway during the trial. This was crucial in the #COVID__19 pandemic where the situation has been constantly evolving
Second, this was the ultimate pragmatic trial (similar to #RECOVERY). Broad eligibility criteria made the findings of this study applicable to the vast majority of patients with #COVID_19 globally. With 405 hospitals in 30 countries recruiting, it reflects the real world closely
Third, trial processes were simplified, adapting remarkably well with the stressed healthcare system. Randomization processes were simple and took just a few minutes. Data collection points were minimized to what was essential. Stunning that they had a 97%+ data completion rate
Fourth, they didn& #39;t fuss much about the sample size! The protocol stated "the larger the number entered, the more accurate the results will be, but numbers entered will depend on how the epidemic develops"! This was practical, considering the uncertainty when the trial started.
Fifth, #SOLIDARITY was a real demonstration of global cooperation in a crisis. Participating countries covered local costs, @WHO covered other study costs, with no extra funding. Collaborators, committee members, data analysts and DMSs charged no costs, and drugs were donated
Finally, 11330 patients were recruited in 197 days between Mar 22nd @ Oct 4th, or a staggering 57.5 patients average recruitment every day! Along with the #RECOVERY trial, these are new benchmarks in therapeutic interventional trial recruitments during pandemics.
The #SOLIDARITY trial tested the following drugs:
#Remdesivir, #HydroxyChloroquine, #Lopinavir & #Interferon
The primary endpoint was mortality; secondary endpoints were need for ventilation & duration of hospitalization
Preplanned subgroup analyses for moderate & severe #COVID19
#Remdesivir, #HydroxyChloroquine, #Lopinavir & #Interferon
The primary endpoint was mortality; secondary endpoints were need for ventilation & duration of hospitalization
Preplanned subgroup analyses for moderate & severe #COVID19
None of the #SOLIDARITY study drugs had an impact on mortality, need for ventilation, or duration of hospitalization. These results held, when analyzed for the whole population, and the prespecified subgroups.
WIth #Remdesivir, the relative risk of death was 0.95 (95% CI, 0.81, 1.11; p=0.5) The Kaplan Meier curves are practically on top of each other. No differences in need for ventilation (295 vs 284); no differences in hospitalization - day 7, 69% vs 59%.
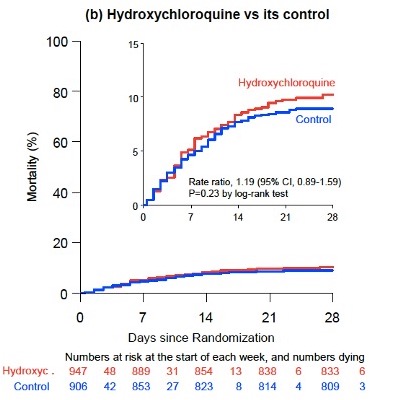
With #HydroxyChloroquine, the relative risk of death was 1.19 (95% CI, 0.89, 1.59; p=0.23). No differences in need for ventilation (75 vs 66); no differences in hospitalization - day 7, 64% vs 54%.
With #Lopinavir, the relative risk of death was 1.00 (95% CI, 0.79, 1.25; p=0.97). No differences in need for ventilation (124 vs 119); no differences in hospitalization - day 7, 68% vs 59%.
With #Interferon, the relative risk of death was 1.16 (95% CI, 0.96, 1.39; p=0.11). No differences in need for ventilation (209 vs 210); no differences in hospitalization - day 7, 55% vs 51%.
Disappointingly, all four drugs tested failed to improve outcomes; yet, this is a definitive trial, which has answered these four important questions reliably. These negative trials are incredibly important. Encouragingly, results are compatible with meta analyses on these drugs.
What #SOLIDARITY (& #RECOVERY) demonstrate conclusively are:
1. It is possible to do large definitive trials, even during the crisis of a pandemic
2. Global collaboration and cooperation is key, and logistic hurdles can be overcome
3. There is no substitute for good science
1. It is possible to do large definitive trials, even during the crisis of a pandemic
2. Global collaboration and cooperation is key, and logistic hurdles can be overcome
3. There is no substitute for good science

 Read on Twitter
Read on Twitter