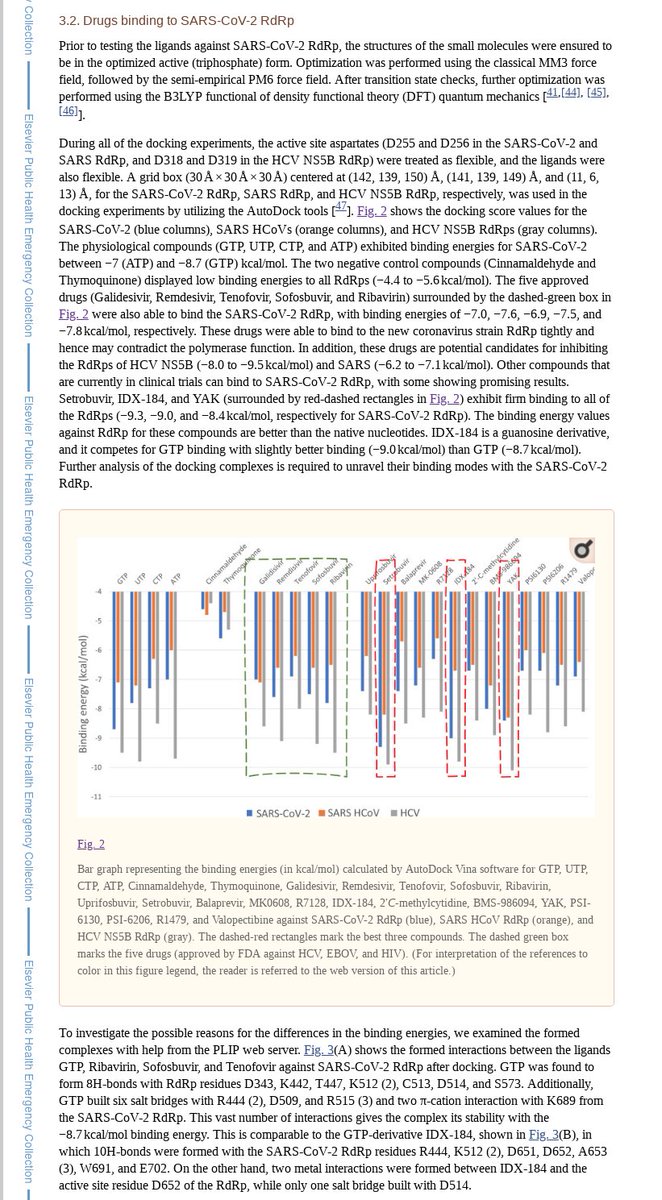
COVID-19 antiviral EC50s expected hittable:
- amodiaquine
- atazanavir
- camostat
- chloroquine
- ciclesonide
- dipyridamole
- doxycycline
- favipiravir
- hydroxychloroquine
- HTCC polymer
- IFN-α 2b
- indomethacin
- itraconazole
- mefloquine
- nelfinavir
- niclosamide
...
Cont.
- amodiaquine
- atazanavir
- camostat
- chloroquine
- ciclesonide
- dipyridamole
- doxycycline
- favipiravir
- hydroxychloroquine
- HTCC polymer
- IFN-α 2b
- indomethacin
- itraconazole
- mefloquine
- nelfinavir
- niclosamide
...
Cont.
...
- nitazoxanide
- opipramol supposedly
- oxyclozanide supposedly
- remdesivir
- rimantidine
- teicoplanin
- tetrandrine supposedly
- nitazoxanide
- opipramol supposedly
- oxyclozanide supposedly
- remdesivir
- rimantidine
- teicoplanin
- tetrandrine supposedly
These are the results from a spreadsheet I made containing 68 candidate drugs of which 51 are being investigated primarily as antivirals or interferon inducers.
The estimates are based on absolute bioavailability, molar mass of the compound (salt form in most cases), typical dosing range in existing indications, and the EC50 and EC90 values from ~20 different papers, of which 9 were high-throughput screens.
Detailed pharmacokinetics studies were cross-checked in some cases, but due to time constraints and varying concentrations within organs vs. plasma, it has not been feasible to confirm each one in detail yet. So that is a potential shortcoming still.
EC90 or 1.5*EC50 (if unknown) as *antivirals* unhittable at normal doses:
- amlodipine
- auranofin
- azithromycin
- baicalin
- benidipine
- bromhexine
- darunavir
- disulfiram
- enoxaparin (as antiviral)
- glycyrrhizin
- isoquercetin
- ivermectin
- lopinavir
...
Cont.
- amlodipine
- auranofin
- azithromycin
- baicalin
- benidipine
- bromhexine
- darunavir
- disulfiram
- enoxaparin (as antiviral)
- glycyrrhizin
- isoquercetin
- ivermectin
- lopinavir
...
Cont.
...
- monensin
- posaconazole
- quercetin
- salinomycin
- tilorone
- umifenovir (Arbidol)
My estimates strongly suggest that lopinavir failed due to underdosing. Bioavailability is poor.
Umifenovir would do better in mesylate form, for the same reason.
- monensin
- posaconazole
- quercetin
- salinomycin
- tilorone
- umifenovir (Arbidol)
My estimates strongly suggest that lopinavir failed due to underdosing. Bioavailability is poor.
Umifenovir would do better in mesylate form, for the same reason.
Neglected to mention 6 that I had rejected out of hand based on prior estimates. So I suppose that& #39;s 74 and 57 respectively.
I see grossly conflicting claims for:
- ambroxol
- nafamostat
- phenazopyridine
As a result, I was unable to make reasonable estimates for these.
Ambroxol is useful for other reasons, mainly lung surfactant.
Nafamostat likely works if camostat does.
Phenazopyridine no idea.
- ambroxol
- nafamostat
- phenazopyridine
As a result, I was unable to make reasonable estimates for these.
Ambroxol is useful for other reasons, mainly lung surfactant.
Nafamostat likely works if camostat does.
Phenazopyridine no idea.
Finally, please note these were just for *repurposing* candidates.
For time reasons, I have not included novel candidate drugs in this listing-- mainly because it would have been much more difficult to estimate bioavailability, and dosing information is often less certain.
For time reasons, I have not included novel candidate drugs in this listing-- mainly because it would have been much more difficult to estimate bioavailability, and dosing information is often less certain.
Novel candidates also tend not to show up in high-throughput screening papers particularly often, for obvious reasons.
I was also unable to make estimates for drugs studied only in silico.
Among the most interesting are more nucleotide analogues, e.g. sofosbuvir:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7102646/
Note">https://www.ncbi.nlm.nih.gov/pmc/artic... ribavirin mentioned: ultimately ineffective, excised by the exoribonuclease.
Need cell culture screens.
Among the most interesting are more nucleotide analogues, e.g. sofosbuvir:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7102646/
Note">https://www.ncbi.nlm.nih.gov/pmc/artic... ribavirin mentioned: ultimately ineffective, excised by the exoribonuclease.
Need cell culture screens.
Among those checked that are not listed in either category above, data was too sparse to make an estimate.
It would be very interesting for a number of reasons if sofosbuvir did indeed already treat this disease.
Someone with access to cheap foreign generics may wish to consider testing it.
Someone with access to cheap foreign generics may wish to consider testing it.
The full spreadsheet is not yet refined enough to post, and it is possible that some implications may change with more data. Perhaps tomorrow.
Pardon limited responsiveness and detail in the interim.
At this point I am convinced a large number of antivirals will be effective.
Pardon limited responsiveness and detail in the interim.
At this point I am convinced a large number of antivirals will be effective.
Finally: I think many people have been drawing the wrong conclusions about in vivo prospects for some candidates over others based on EC50/EC90 data, due to not adjusting properly for molar mass, bioavailability, and tolerable dosing.
For example:
Lopinavir performs well in EC50 terms, but has poor bioavailability and limited dosing.
Favipiravir looks impractical at first glance in the charts, but it has a molar mass of only 157g/mol, no salt anions, 97% bioavailability, and is tolerated at >1600mg/dose.
Lopinavir performs well in EC50 terms, but has poor bioavailability and limited dosing.
Favipiravir looks impractical at first glance in the charts, but it has a molar mass of only 157g/mol, no salt anions, 97% bioavailability, and is tolerated at >1600mg/dose.
For those that appear hittable, usually the dosing is toward the upper range of current practice for the original indication-- with a few conspicuous exceptions. More on that a bit later.
And in comparative studies, usually EC50s for SARS CoVs were much higher than for HCoVs.
And in comparative studies, usually EC50s for SARS CoVs were much higher than for HCoVs.
And for clarification: I absolutely believe there is a meaningful difference between only dosing high enough to get 10-30% inhibition, versus smashing through the EC90 and beyond.
Reluctance to halt viral replication early with a multi-mechanism approach will lead to failure.
Reluctance to halt viral replication early with a multi-mechanism approach will lead to failure.
Lopinavir also has a tiny volume of distribution-- about 17 liters. That means it tends to stay trapped in the blood plasma, mostly bound to albumin.
Hydroxychloroquine has an enormous volume of distribution: 733L. It concentrates in the lungs, conveniently enough.
Hydroxychloroquine has an enormous volume of distribution: 733L. It concentrates in the lungs, conveniently enough.
Atazanavir: 78L, another reason to believe it will likely work better than lopinavir.
Nelfinavir: 2-7L/kg, or 140-490L for a 70kg patient. On top of superior bioavailability and the best EC50 of all the major viral protease inhibitors.
The question is: why lopinavir at all?
Nelfinavir: 2-7L/kg, or 140-490L for a 70kg patient. On top of superior bioavailability and the best EC50 of all the major viral protease inhibitors.
The question is: why lopinavir at all?
The more I look at this, the more it appears some initial choices ignored crucial considerations of basic pharmacokinetics.

 Read on Twitter
Read on Twitter