Proposed COVID-19 Pathophysiology [Part 2 of 3]:
Enter the magnificent macrophage.
What do #COVID-19 and hemophagocytic lymphohistiocytosis ( #HLH) potentially have in common?
...let’s continue our journey onward into the cytokine storm.
Enter the magnificent macrophage.
What do #COVID-19 and hemophagocytic lymphohistiocytosis ( #HLH) potentially have in common?
...let’s continue our journey onward into the cytokine storm.
First, let’s briefly summarize what we covered before:
What mechanism may be partly responsible for heterogeneity of COVID-19 disease phenotype as well as its severity?
What mechanism may be partly responsible for heterogeneity of COVID-19 disease phenotype as well as its severity?
Correct! Antibody-dependent enhancement (ADE) is the result of producing sub-neutralizing antibodies which paradoxically enhance the rate of viral replication and severity of viremia.
Part of the vascular injury seen in COVID-19 may be a product of which of the following:
All of the above may be at play in the generation of the immune response and subsequent tissue damage:
What happens to immune complexes after deposition?
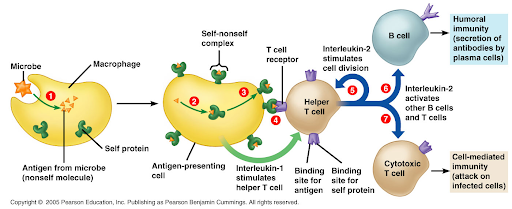
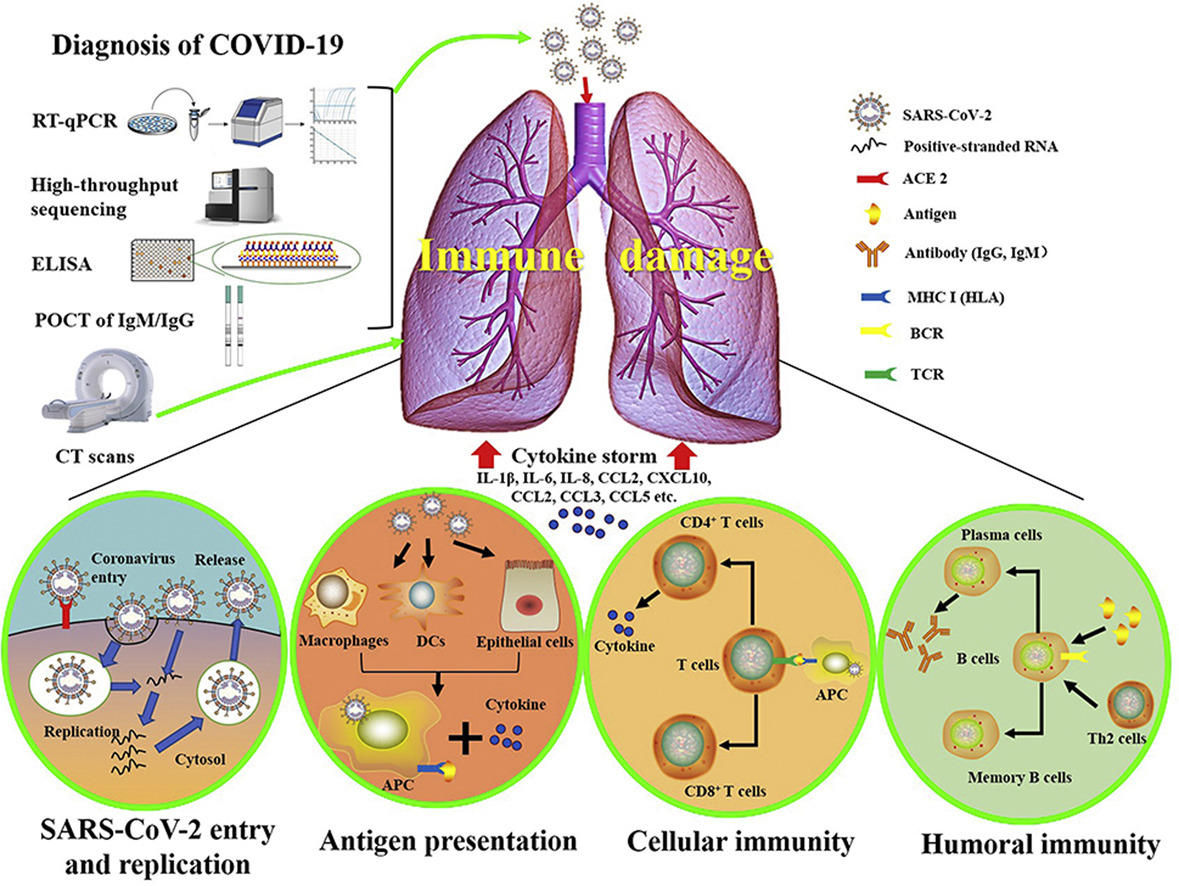
Macrophage (M0) phagocytosis→ antigen presentation → cytokine release → TH1 response → CD8+ T and B-cell ab response
What happens to immune complexes after deposition?
Macrophage (M0) phagocytosis→ antigen presentation → cytokine release → TH1 response → CD8+ T and B-cell ab response
Of note, the severity of this response is proportional to the amount of antibody-antigen complex present.
Now for those that may not know, what is hemophagocytic lymphohistiocytosis (HLH)?
Now for those that may not know, what is hemophagocytic lymphohistiocytosis (HLH)?
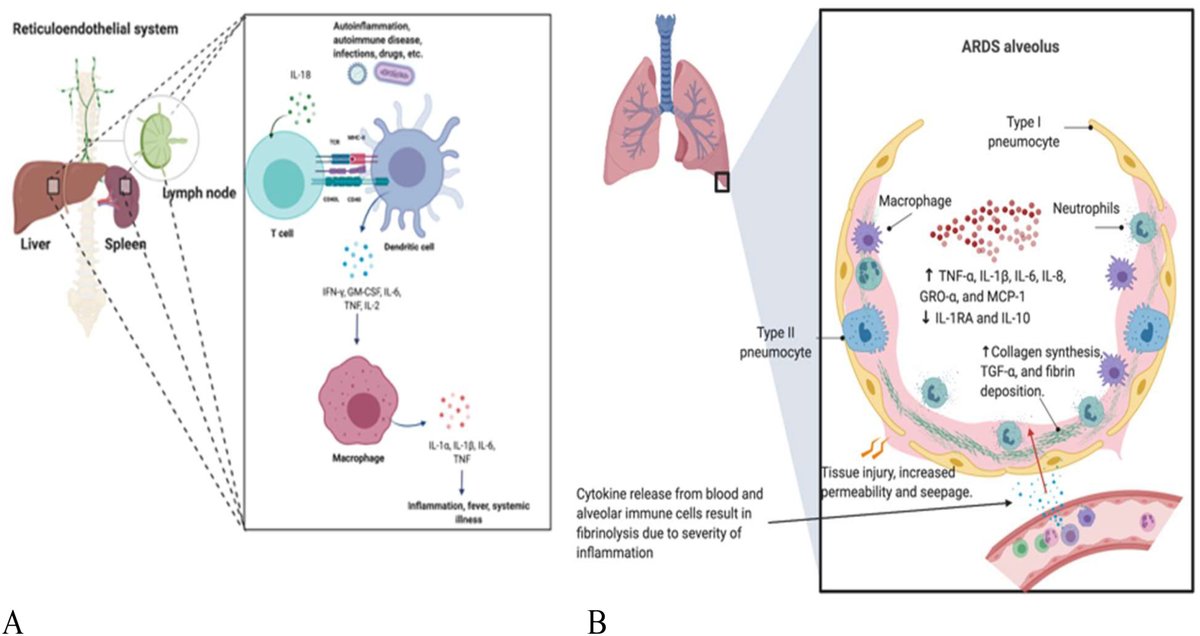
HLH is a M0 hyperactivation syndrome!
It results in catastrophic cytokine production (“cytokine storm”)
It also leads to hemophagocytosis, where M0s ultimately begin consuming the body’s own red blood cells / WBCs to a pathological level (see below)
It results in catastrophic cytokine production (“cytokine storm”)
It also leads to hemophagocytosis, where M0s ultimately begin consuming the body’s own red blood cells / WBCs to a pathological level (see below)
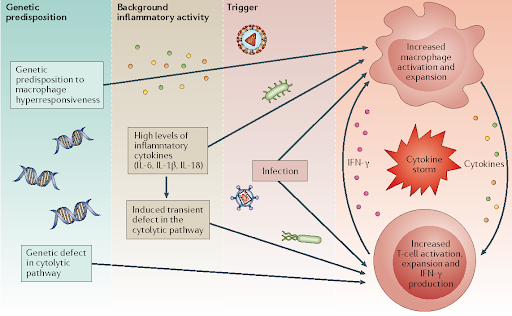
Incredibly simply put, it is thought to be stimulated by a multi-hit process, where a pre-existing condition such as cancer, autoimmune disease, or infection meets a triggering event, often severe infection.
(Its full pathophysiology will be a tweetorial for another day.)
(Its full pathophysiology will be a tweetorial for another day.)
What is the hallmark laboratory finding (often dramatic) in HLH?
Ferritin is often considerably elevated, with levels >3000 strongly suggestive of developing HLH.
Where does this marked rise ferritin come from in HLH?
Where does this marked rise ferritin come from in HLH?
M0s serve as master regulators of iron metabolism - every iron molecule in your body passes through them.
Ferritin is released from M0s to help facilitate the starvation of bacterial pathogens of free iron (hence why it is an acute phase reactant / part of the cytokine response)
Ferritin is released from M0s to help facilitate the starvation of bacterial pathogens of free iron (hence why it is an acute phase reactant / part of the cytokine response)
Generally speaking, what cytokines are produced by M0s?
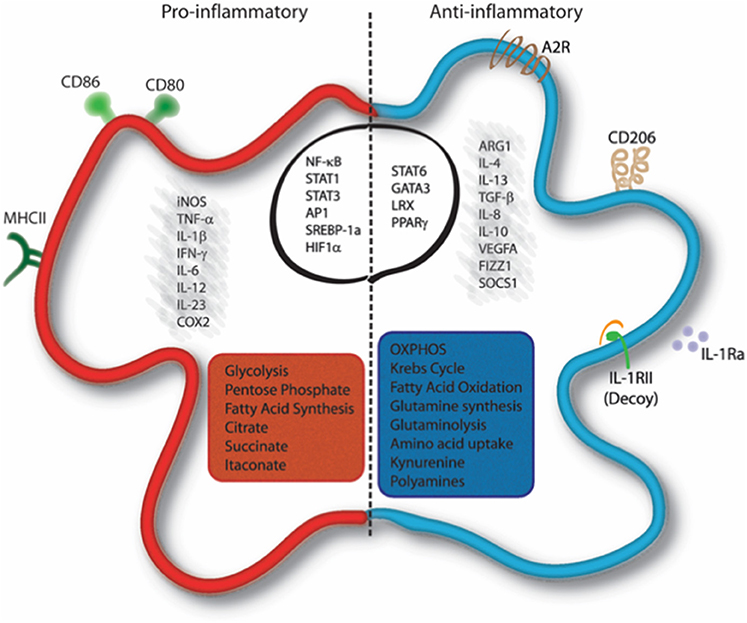
Well...it depends on their polarization!
M0s wear multiple hats. Their function depends on the surrounding environment and physiological need.
They can act both as promoters or regulators of inflammation.
Well...it depends on their polarization!
M0s wear multiple hats. Their function depends on the surrounding environment and physiological need.
They can act both as promoters or regulators of inflammation.
The polarization of macrophages is a complex process, but is simplified as follows:
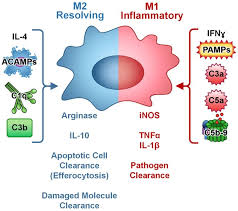
M0s have two main phenotypes - M1 and M2. Think of them as the ninja janitors ("ninjanitors") of your body:
M1 = Pro-inflammatory ninja!
M2 = Anti-inflammatory janitor!
M0s have two main phenotypes - M1 and M2. Think of them as the ninja janitors ("ninjanitors") of your body:
M1 = Pro-inflammatory ninja!
M2 = Anti-inflammatory janitor!
M1 release of pro-inflammatory cytokines, namely:
IL-1 → Fever + acute phase response + IL-2/TH2 response
IL-6 → Fever + acute phase response + antibody production
TNF-a → Further M0 activation + inc. cytokine production
IFN-y → Cell priming + M0 & T-cell activation
IL-1 → Fever + acute phase response + IL-2/TH2 response
IL-6 → Fever + acute phase response + antibody production
TNF-a → Further M0 activation + inc. cytokine production
IFN-y → Cell priming + M0 & T-cell activation
M2 produce anti-inflammatory cytokines (i.e. IL-4, IL-10, and TGF-beta) that facilitate tissue repair & promote angiogenesis.
Do parts of this seem familiar? They should.
The cytokine profile seen in HLH mirrors what is seen in COVID-19 because both create a robust M1 response!
Do parts of this seem familiar? They should.
The cytokine profile seen in HLH mirrors what is seen in COVID-19 because both create a robust M1 response!
And what& #39;s the purpose of an M1 response?
1. Cull other M0s, T-cells, and B-cells! This is to promote a humoral response, generating antibodies. It also
2. Self-promotes itself, creating a positive feedback cycle which continues until it
3. Eradicates the pathogen
1. Cull other M0s, T-cells, and B-cells! This is to promote a humoral response, generating antibodies. It also
2. Self-promotes itself, creating a positive feedback cycle which continues until it
3. Eradicates the pathogen
Negative feedback of this M1 response depends on generation of a humoral response and removal of the inciting stimulus.
T-cells play an equally important role in viral regulation as well as the generation of an appropriate B-cell antibody response.
T-cells play an equally important role in viral regulation as well as the generation of an appropriate B-cell antibody response.
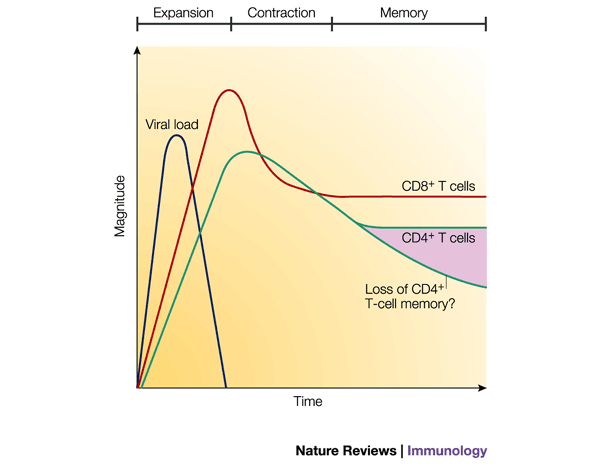
...but what happens if your lymphocyte response is hindered?
We know that the severity of lymphopenia is correlated with the severity of disease in COVID-19.
( https://twitter.com/Leo_ReapDO/status/1244997878247981057?s=20)
Withhttps://twitter.com/Leo_ReapD... class="Emoji" style="height:16px;" src=" https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">lymphs, regulating both worsening viremia and the M1 response would be more difficult.
We know that the severity of lymphopenia is correlated with the severity of disease in COVID-19.
( https://twitter.com/Leo_ReapDO/status/1244997878247981057?s=20)
With
Thus, viral-mediated lymphocyte destruction may contribute to the HLH-like phenotype:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> T and B-cells = blunted humoral response:
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> T and B-cells = blunted humoral response:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers->
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers->  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response ->
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response ->  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremia
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremia
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback +
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback +  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation
#f0005">https://www.sciencedirect.com/science/article/pii/S1568997220300926 #f0005">https://www.sciencedirect.com/science/a...
#f0005">https://www.sciencedirect.com/science/article/pii/S1568997220300926 #f0005">https://www.sciencedirect.com/science/a...
As loss of control of viremia occurs, more viral particles would be taken up into M0s, further driving M1 phenotype and a pro-inflammatory response!
Note: All positive feedback cycles end in an explosion.
In this case, the explosion = widespread pulmonary inflammation / injury!
Note: All positive feedback cycles end in an explosion.
In this case, the explosion = widespread pulmonary inflammation / injury!
TL;DR:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">COVID-19 and HLH share considerable similarities in their cytokine patterns, reflecting M1 hyperactivation.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">COVID-19 and HLH share considerable similarities in their cytokine patterns, reflecting M1 hyperactivation.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Lymphopenia may be due to direct viral invasion; this correlates with disease severity.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Lymphopenia may be due to direct viral invasion; this correlates with disease severity.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Loss of TH1 / T-reg response may lead to constitutive M1 activation.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Loss of TH1 / T-reg response may lead to constitutive M1 activation.
Additional reading if interested:
#f0005">https://www.sciencedirect.com/science/article/pii/S1568997220300926 #f0005
https://www.sciencedirect.com/science/a... href=" https://www.medrxiv.org/content/10.1101/2020.03.24.20042655v1.full.pdf
https://www.medrxiv.org/content/1... href=" https://www.ncbi.nlm.nih.gov/pubmed/32217834
https://www.ncbi.nlm.nih.gov/pubmed/32... href="https://twtext.com//hashtag/medtwitter"> #medtwitter #covid4MDs #COVID19 #hematology #pathophysiology #MedEdForum #FOAMcovid #FOAMed #COVID #coronavirus
Part 3 to come soon!
#f0005">https://www.sciencedirect.com/science/article/pii/S1568997220300926 #f0005
Part 3 to come soon!
Further points of clarification here, thanks to a great discussion by @angrybiomed1: https://twitter.com/angrybiomed1/status/1250831889956343808?s=20">https://twitter.com/angrybiom...

 Read on Twitter
Read on Twitter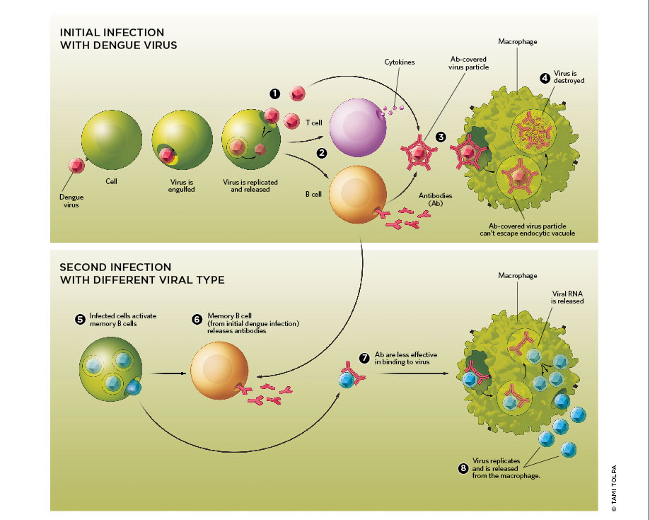


 T and B-cells = blunted humoral response:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers-> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response -> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremiahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback + https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation https://www.sciencedirect.com/science/a..." title="Thus, viral-mediated lymphocyte destruction may contribute to the HLH-like phenotype:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> T and B-cells = blunted humoral response:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers-> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response -> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremiahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback + https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation https://www.sciencedirect.com/science/a..." class="img-responsive" style="max-width:100%;"/>
T and B-cells = blunted humoral response:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers-> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response -> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremiahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback + https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation https://www.sciencedirect.com/science/a..." title="Thus, viral-mediated lymphocyte destruction may contribute to the HLH-like phenotype:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> T and B-cells = blunted humoral response:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">Ab titers-> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> sub-neutralizing antibody response -> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> viremiahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> Negative feedback + https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow">viremia --> M1 hyperactivation https://www.sciencedirect.com/science/a..." class="img-responsive" style="max-width:100%;"/>


