Proposed COVID-19 Pathophysiology [Part 1 of 3]
What do #COVID-19 and #Dengue fever potentially have in common?
The answers have significant potential clinical implications in COVID-19.
Buckle up. It& #39;s a long one.
Let’s take a hematological walk on the zoonotic side.
What do #COVID-19 and #Dengue fever potentially have in common?
The answers have significant potential clinical implications in COVID-19.
Buckle up. It& #39;s a long one.
Let’s take a hematological walk on the zoonotic side.
Dengue is a flavivirus that is called “breakbone fever”, a nod to bone-breaking pains that occur during the infection.
There are four serotypes of the dengue virus.
Infection with one serotype does not fully protect one from contracting a different serotype.
There are four serotypes of the dengue virus.
Infection with one serotype does not fully protect one from contracting a different serotype.
True or false: The second or later infection with dengue is often much more dangerous.
It’s true! The first infection with Dengue often causes mild to moderate disease, whereas the second infection can create a life-threatening hemorrhagic fever.
The mechanism for why is fascinating.
The mechanism for why is fascinating.
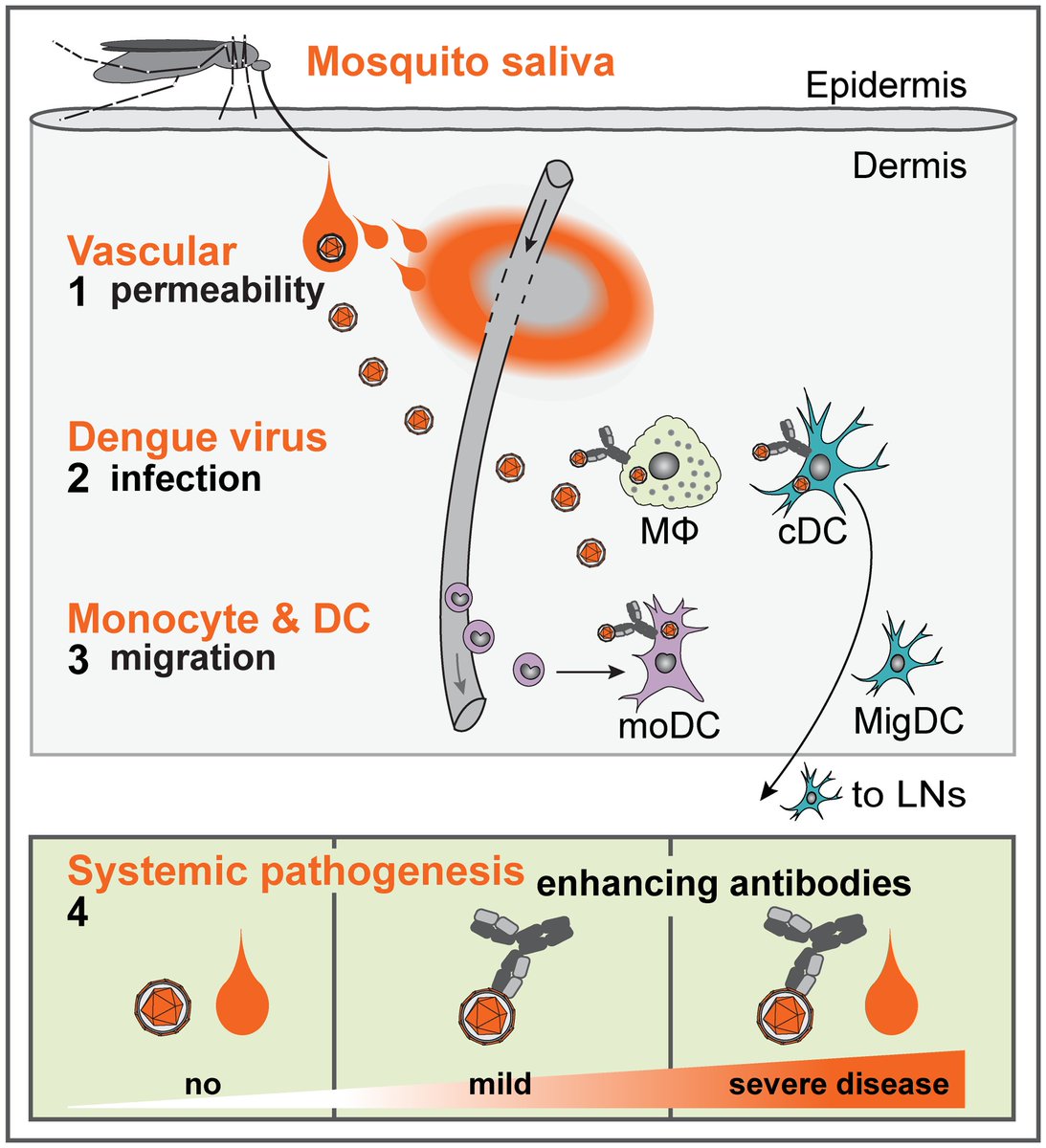
Dengue initially is inoculated into the skin by the Aedes mosquito, where it infects skin dendritic cells.
It is then transported to nearby lymph nodes, ultimately creating a neutralizing antibody-mediated response.
...dengue has a trick up its sleeve, though.
What is it?
It is then transported to nearby lymph nodes, ultimately creating a neutralizing antibody-mediated response.
...dengue has a trick up its sleeve, though.
What is it?
The antibody response is both a blessing and a curse.
With first infection, the virus is effectively neutralized and confers strong protection from that particular serotype.
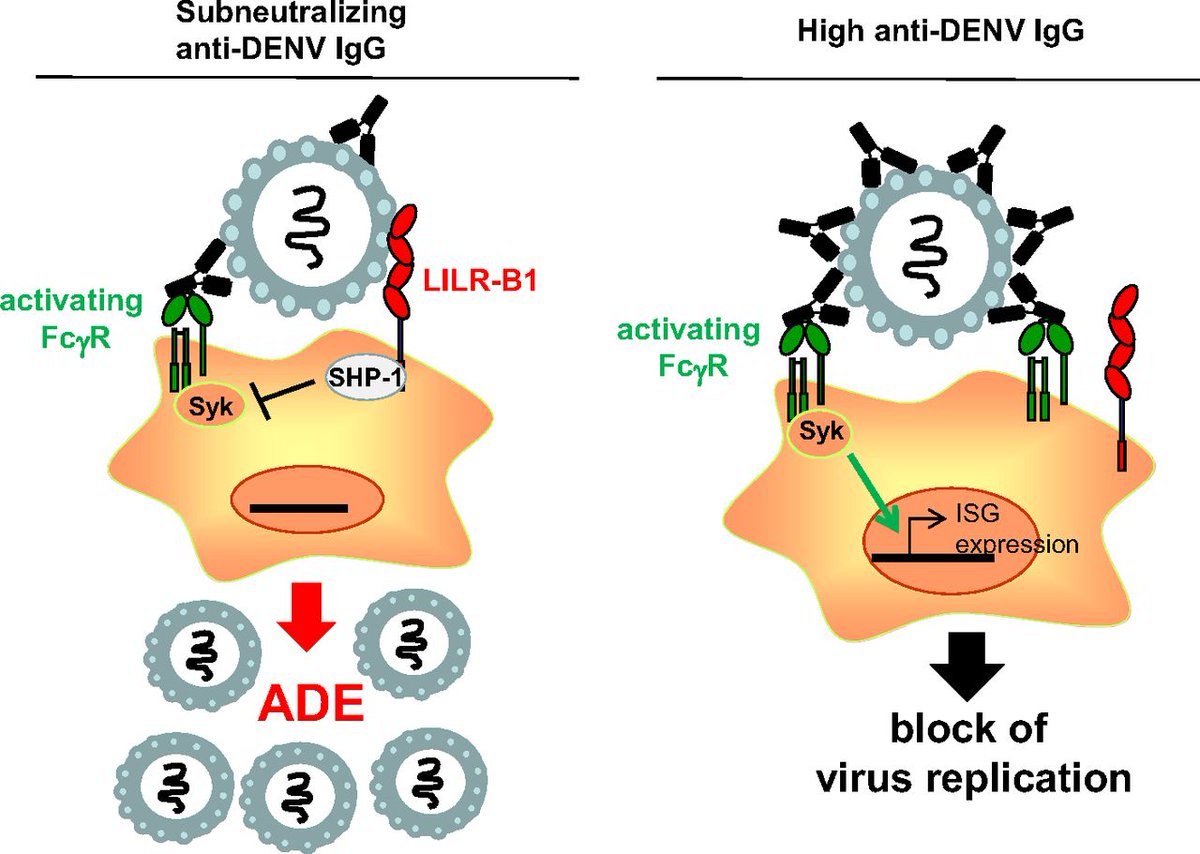
However a weak antibody response, called a sub-neutralizing response, occurs toward other serotypes.
With first infection, the virus is effectively neutralized and confers strong protection from that particular serotype.
However a weak antibody response, called a sub-neutralizing response, occurs toward other serotypes.
When a patient is infected with another serotype, it doesn’t get rid of the virus - it does the exact opposite:
Sub-neutralizing antibodies facilitate its uptake into macrophages / dendritic cells!
This paradoxically hastens viral replication and viremia!
Sub-neutralizing antibodies facilitate its uptake into macrophages / dendritic cells!
This paradoxically hastens viral replication and viremia!
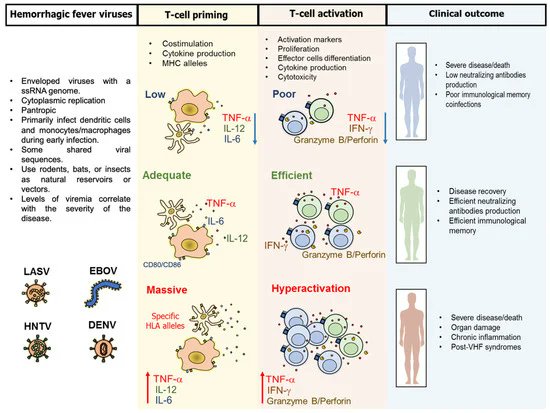
This creates widespread T-cell and macrophage activation.
Cytokine storm ensues.
Vascular endothelial dysfunction occurs, leading to a prothrombotic state.
Local mast cell granule release leads to further capillary leak.
Eventually, hemorrhagic shock ensues.
Cytokine storm ensues.
Vascular endothelial dysfunction occurs, leading to a prothrombotic state.
Local mast cell granule release leads to further capillary leak.
Eventually, hemorrhagic shock ensues.
This phenomenon, known as antibody-dependent enhancement (ADE), is a reverse-Goldilocks scenario:
Make an isolated strong neutralizing antibody? You rapidly clear the virus.
Make little to no antibody? You tend not to manifest severe disease or hemorrhagic complications.
Make an isolated strong neutralizing antibody? You rapidly clear the virus.
Make little to no antibody? You tend not to manifest severe disease or hemorrhagic complications.
...but make a weak, sub-neutralizing antibody?
You get rapid-onset, accelerated viremia with the development of hemorrhagic shock.
ADE is not exclusive to Dengue. It is seen in several other types of viruses - particularly those that cause hemorrhagic fever - such as Ebola.
You get rapid-onset, accelerated viremia with the development of hemorrhagic shock.
ADE is not exclusive to Dengue. It is seen in several other types of viruses - particularly those that cause hemorrhagic fever - such as Ebola.
So how does all this relate to SARS CoV2?
Similar to Dengue, there are 5 non-SARS coronavirus serotypes, and we are quick to forget they compose the vast majority of coronavirus infections.
They tend to cause mild URI symptoms and readily circulate through the human population.
Similar to Dengue, there are 5 non-SARS coronavirus serotypes, and we are quick to forget they compose the vast majority of coronavirus infections.
They tend to cause mild URI symptoms and readily circulate through the human population.
Antibodies to non-SARS coronaviruses correlate with age.
Per a Finnish study:
<2 yo = no measurable coronavirus antibodies
2-6 yo = a rapid increase in antibody prevalence
Antibody levels continue to increase until 10-14 years, likely the result of cross-serotype boosting.
Per a Finnish study:
<2 yo = no measurable coronavirus antibodies
2-6 yo = a rapid increase in antibody prevalence
Antibody levels continue to increase until 10-14 years, likely the result of cross-serotype boosting.
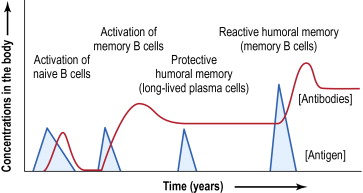
Memory B-cells often persist for decades. However, immunity wanes (one reason elderly at risk for infection).
So why aren’t we seeing severe COVID-19 so much in children?
One answer may be because children haven’t yet developed high levels of sub-neutralizing antibodies.
So why aren’t we seeing severe COVID-19 so much in children?
One answer may be because children haven’t yet developed high levels of sub-neutralizing antibodies.
Not convinced? Let& #39;s continue on.
Like Dengue, coronavirus antibodies are known to cross-react between serotypes.
Antibody dependent enhancement has been shown to occur in human coronaviruses and is not a novel concept:
https://www.ncbi.nlm.nih.gov/pubmed/32092539
https://www.ncbi.nlm.nih.gov/pubmed/32... href=" #ref-CR10">https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-11-82 #ref-CR10">https://virologyj.biomedcentral.com/articles/...
Like Dengue, coronavirus antibodies are known to cross-react between serotypes.
Antibody dependent enhancement has been shown to occur in human coronaviruses and is not a novel concept:
https://www.ncbi.nlm.nih.gov/pubmed/32092539
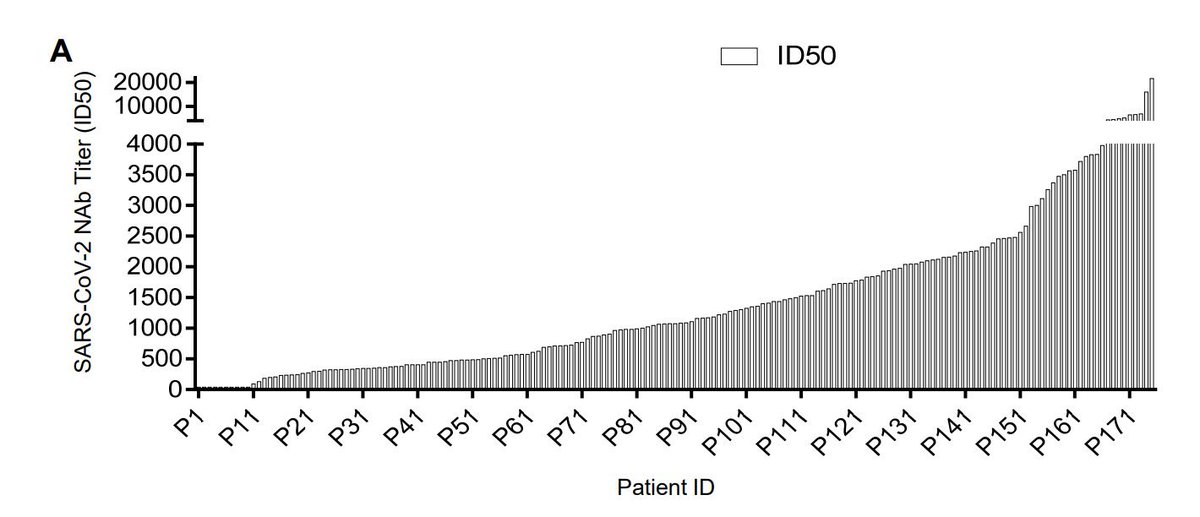
Furthermore, like other coronaviruses, the antibody response to SARS Cov2 is widely varied.
This exacerbates the heterogeneity of the disease phenotype in COVID19, as low antibody titers would potentially lead to lesser disease severity and vice-versa (Wu et. al, 2020):
This exacerbates the heterogeneity of the disease phenotype in COVID19, as low antibody titers would potentially lead to lesser disease severity and vice-versa (Wu et. al, 2020):
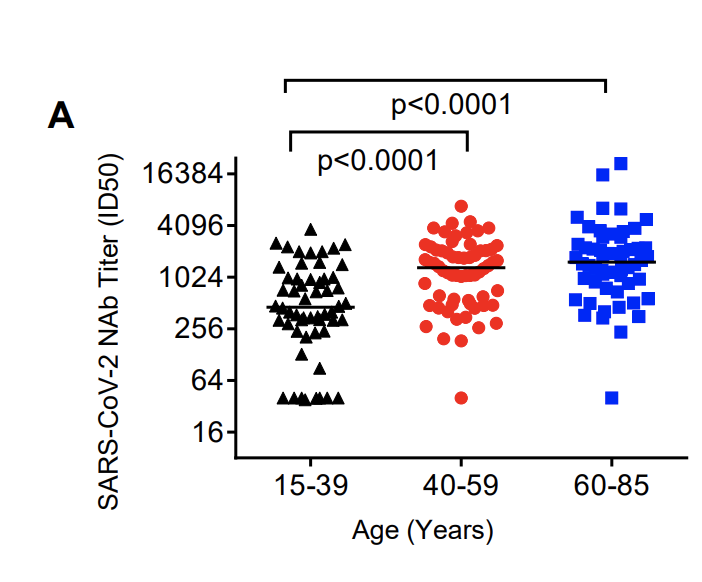
Neutralizing antibody titers in COVID-19 are associated with age.
The older the individual, the greater the degree of neutralizing antibody titers:
The older the individual, the greater the degree of neutralizing antibody titers:
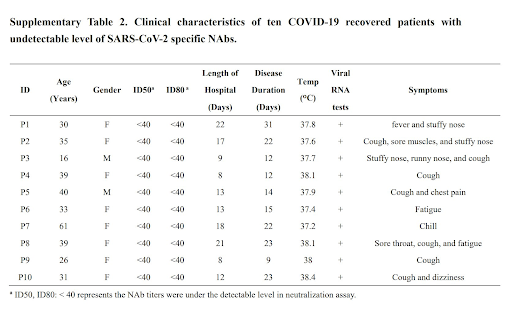
Even further still, those with undetectable antibody levels have a very mild course of illness, explaining why severe disease may not be seen in younger individuals or those with little immunity!
These data point toward an antibody-mediated pathogenesis of COVID-19:
These data point toward an antibody-mediated pathogenesis of COVID-19:
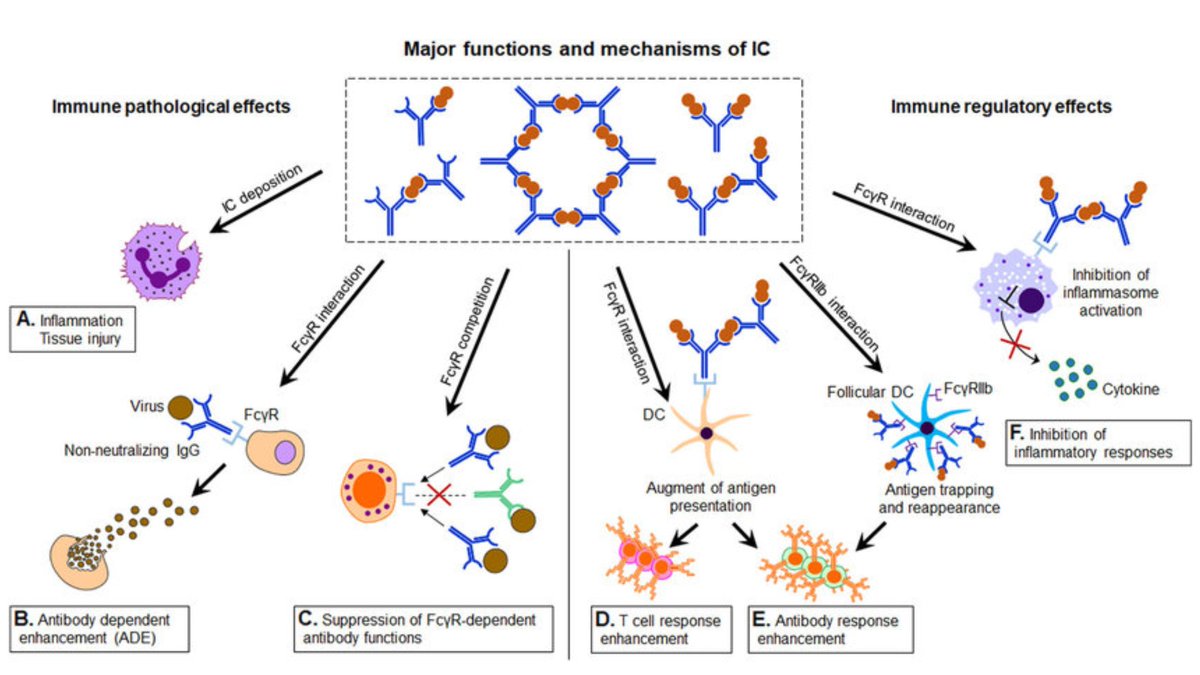
Type III immune complex deposition fits with potential pathophysiology for the following reasons:
(For those that don& #39;t remember: Type III = antigen/antibody complex formation --> capillary interstitial deposition --> immune activation --> endothelial damage [see below])
(For those that don& #39;t remember: Type III = antigen/antibody complex formation --> capillary interstitial deposition --> immune activation --> endothelial damage [see below])
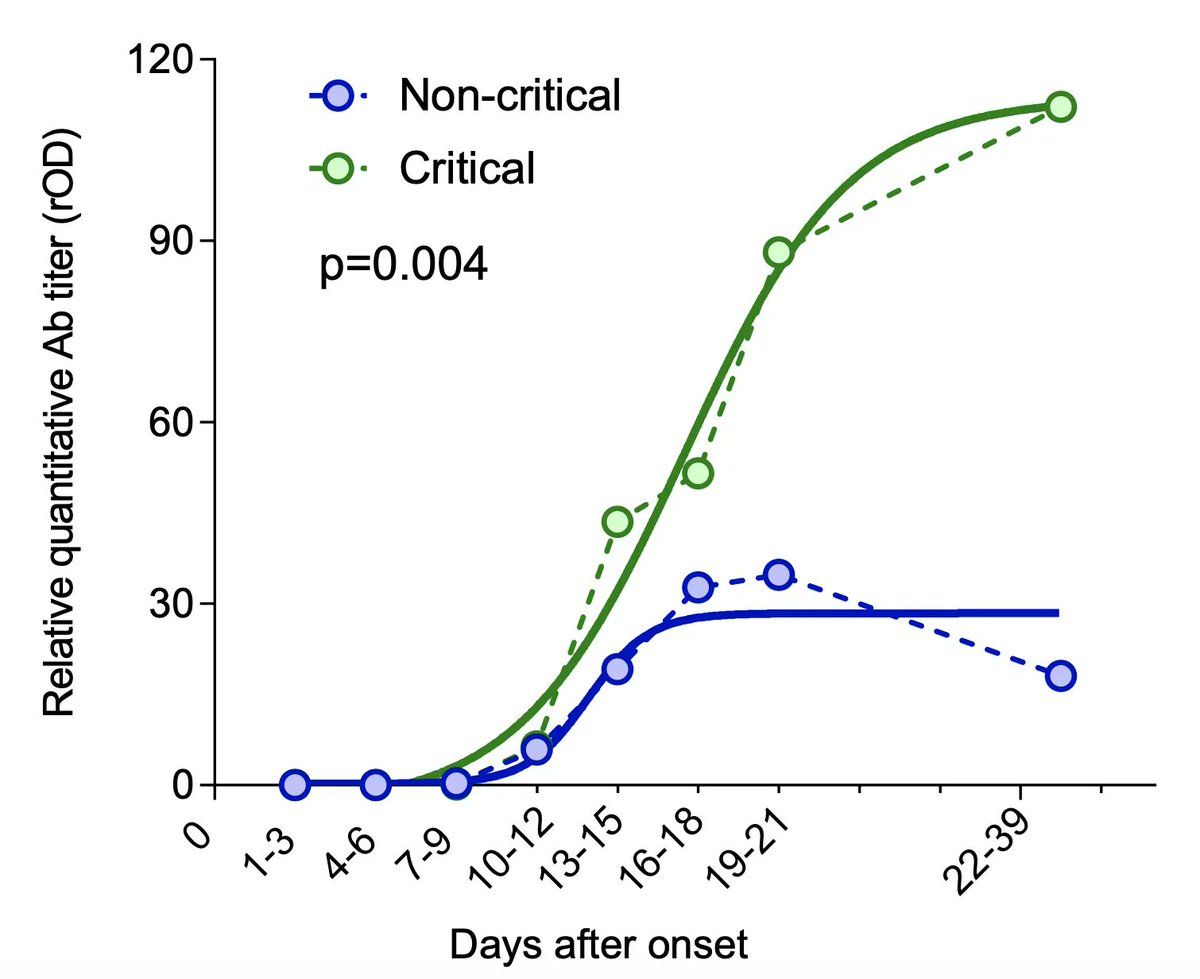
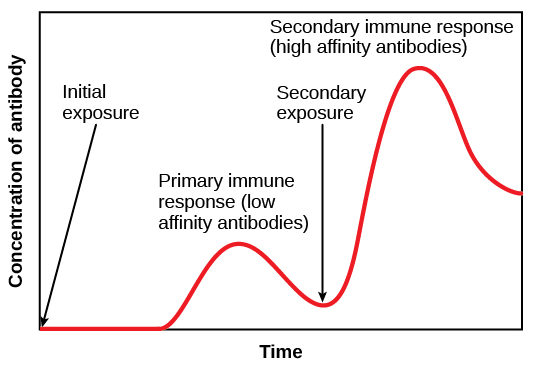
1. Type III hypersensitivity reactions typically develop ~6 h after exposure to antigen.
This is the timeframe we are often seeing patients rapidly decompensate in (2L -> 15L -> intubation), usually around day 7 to 14, when an antibody response would be reaching a peak (below)
This is the timeframe we are often seeing patients rapidly decompensate in (2L -> 15L -> intubation), usually around day 7 to 14, when an antibody response would be reaching a peak (below)
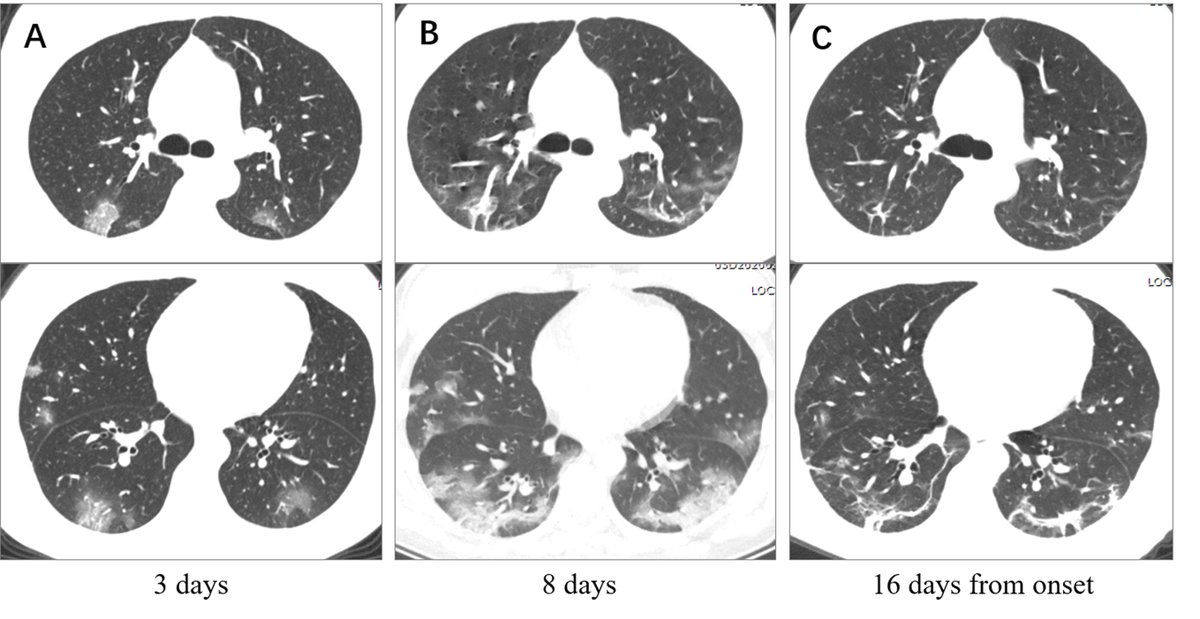
2. Basal lung = where perfusion is greatest
Peripheral lung = where capillary networks are richest.
Immune complexes most readily deposit within the interstitium of capillaries --> lymphohistiocytic response.
...this potentially explains the predominant CT findings in COVID-19.
Peripheral lung = where capillary networks are richest.
Immune complexes most readily deposit within the interstitium of capillaries --> lymphohistiocytic response.
...this potentially explains the predominant CT findings in COVID-19.
3. Lung injury in COVID-19 does not behave like classical ARDS.
Patients are poor to oxygenate but have adequate lung compliance, especially early on.
This fits with interstitial edema > intra-alveolar edema and also explains why high PEEP is often needed in these patients.
Patients are poor to oxygenate but have adequate lung compliance, especially early on.
This fits with interstitial edema > intra-alveolar edema and also explains why high PEEP is often needed in these patients.
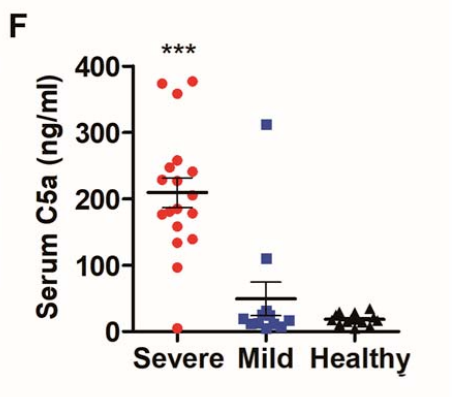
4. Immune complex deposition leads to complement activation.
Complement over-activation, particularly within the lung tissue, is seen in the severe phase of COVID19.
Notably, it is not seen much in the early phase.
Complement over-activation, particularly within the lung tissue, is seen in the severe phase of COVID19.
Notably, it is not seen much in the early phase.
5. Memory B cells have been shown to persist in the lung following pulmonary infection with various respiratory viruses.
An antibody response occurring within the lungs would lead to the greatest degree of intrapulmonary immune complex deposition and damage.
An antibody response occurring within the lungs would lead to the greatest degree of intrapulmonary immune complex deposition and damage.
6. Distant immune complex spread would be variable may partly explain the heterogeneity of other organ damage:
Renal deposition --> AKI / ARF
Muscle capillary bed --> rhabdomyolysis
GI tract --> colitis
Cardiac --> coronary arteritis
CNS --> hemorrhagic necrotizing encephalitis
Renal deposition --> AKI / ARF
Muscle capillary bed --> rhabdomyolysis
GI tract --> colitis
Cardiac --> coronary arteritis
CNS --> hemorrhagic necrotizing encephalitis
TL;DR:
1. Non-SARS coronaviruses are ubiquitous
2. Antibody levels parallel age
3. Sub-neutralizing antibodies are key, as they enhance viremia and disease severity
4. Ab/ag complex deposition may drive severe disease phenotype via type III / IV hypersensitivity rxns
Part 2:
1. Non-SARS coronaviruses are ubiquitous
2. Antibody levels parallel age
3. Sub-neutralizing antibodies are key, as they enhance viremia and disease severity
4. Ab/ag complex deposition may drive severe disease phenotype via type III / IV hypersensitivity rxns
Part 2:

 Read on Twitter
Read on Twitter