Nice visual overview, for those who haven& #39;t seen it yet. When researchers discuss interfering with the function of the SARS-CoV-2 Spike protein, they are referring to the knobby proteins protruding from the viral envelope, shown here in red: https://elemental.medium.com/what-the-coronavirus-image-youve-seen-a-million-times-really-shows-3d8de7e3eb1f">https://elemental.medium.com/what-the-...
SARS-CoV-2 Spike proteins have a greater binding affinity for their target receptor than their equivalent from the SARS-CoV virus that caused the first major SARS epidemic.
As noted in earlier discussions, it predominantly targets the human ACE-2 receptor: https://www.nature.com/articles/s41564-020-0688-y">https://www.nature.com/articles/...
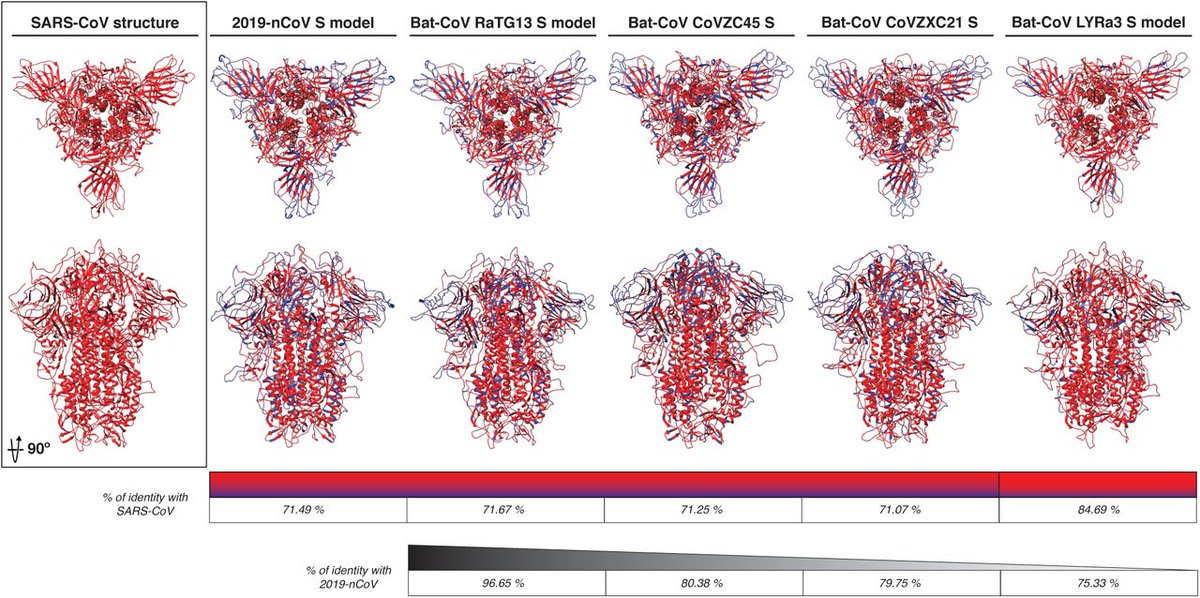
Spike proteins from related coronaviruses:
https://www.biorxiv.org/content/10.1101/2020.02.10.942185v1.full">https://www.biorxiv.org/content/1...
https://www.biorxiv.org/content/10.1101/2020.02.10.942185v1.full">https://www.biorxiv.org/content/1...
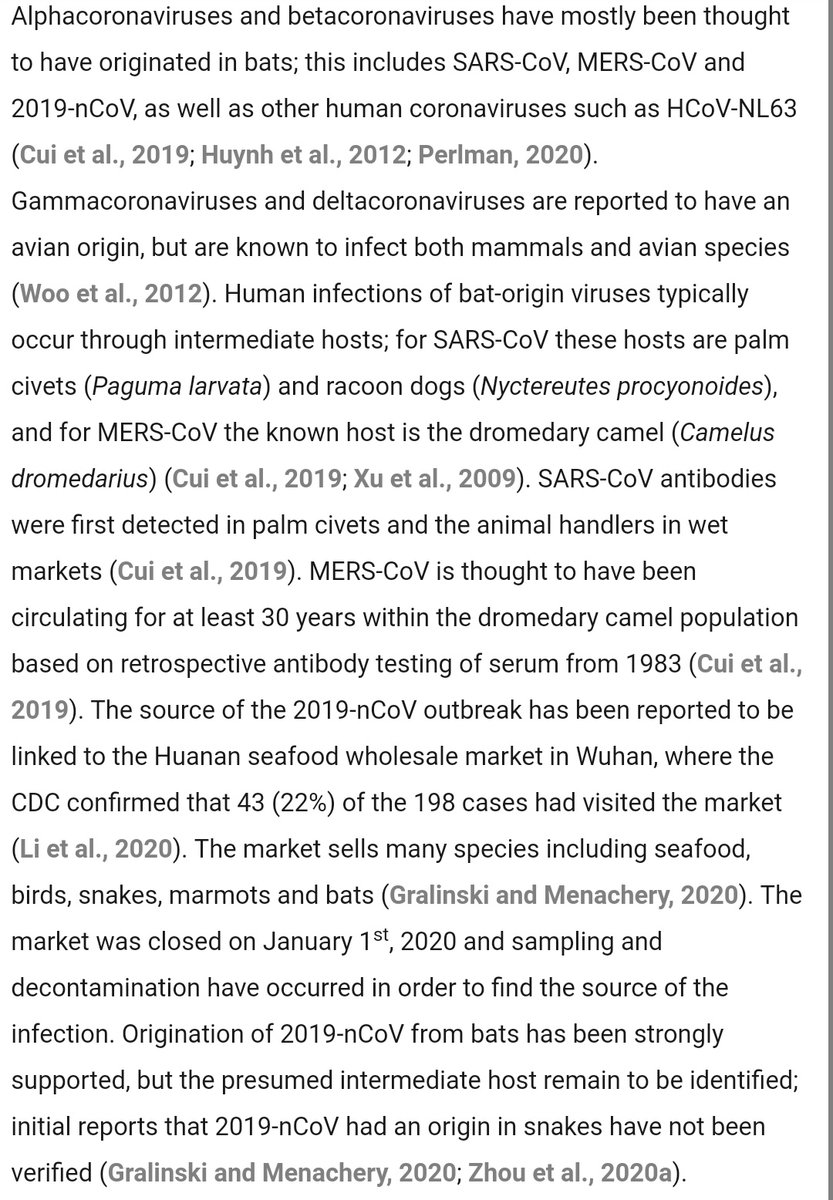
2019-nCov (SARS-CoV-2) is a betacoronavirus.
These generally infect mostly bats as their reservoir species, with some intermediate propagation through civets and raccoon dogs.
These generally infect mostly bats as their reservoir species, with some intermediate propagation through civets and raccoon dogs.
One tip of the S protein, domain S1, binds to the ACE-2 receptor.
After binding, the S2 domain behind it ends up being cleaved upstream by proteases expressed by the host, producing a fusion protein that fuses the virus into the cell membrane.
After binding, the S2 domain behind it ends up being cleaved upstream by proteases expressed by the host, producing a fusion protein that fuses the virus into the cell membrane.
Here is what that looks like, for a related betacoronavirus.
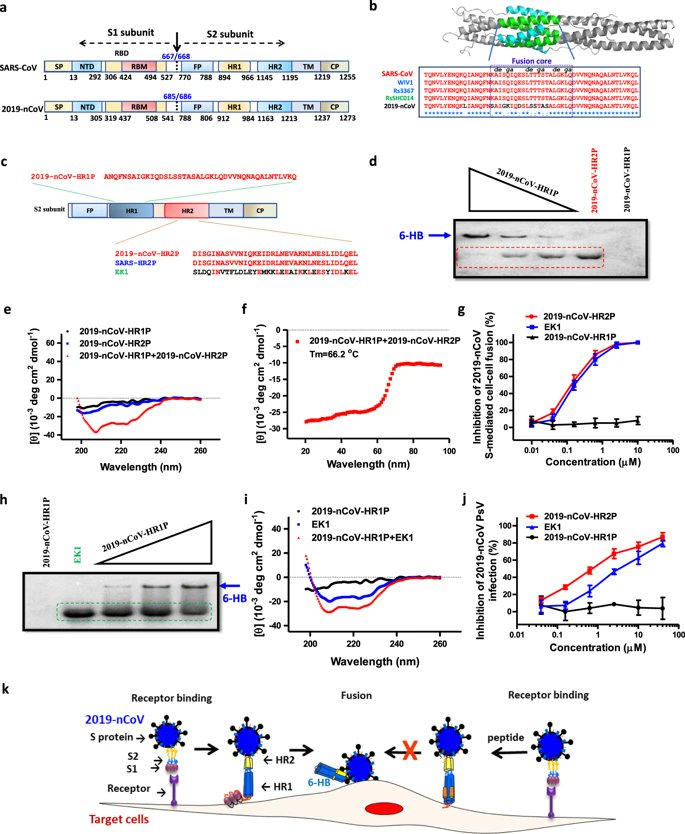
S2 sits behind S1 before being cleaved by the host proteases.
The combined Spike protein is only metastable in its trimerized protruding configuration. Cleavage allows it to open up.
https://www.nature.com/articles/nature17200">https://www.nature.com/articles/...
S2 sits behind S1 before being cleaved by the host proteases.
The combined Spike protein is only metastable in its trimerized protruding configuration. Cleavage allows it to open up.
https://www.nature.com/articles/nature17200">https://www.nature.com/articles/...
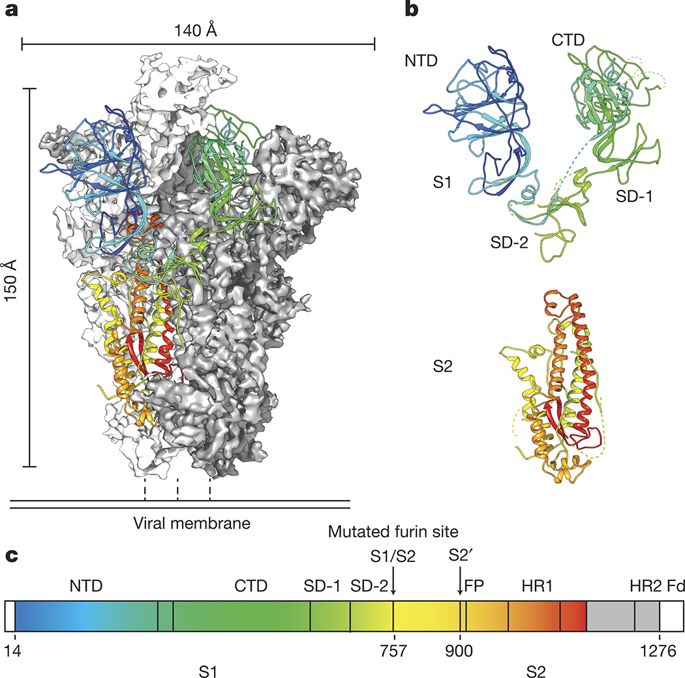
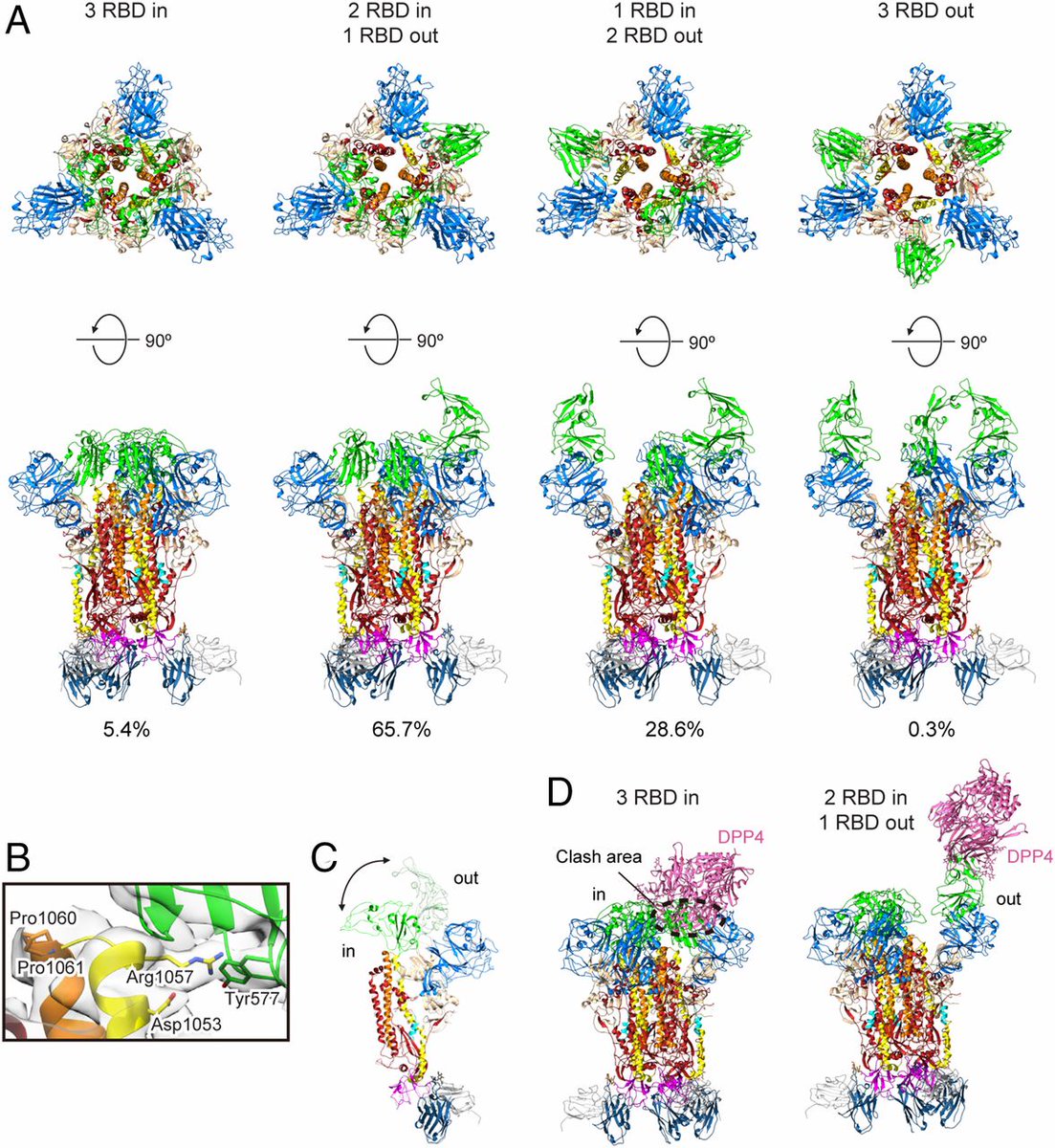
Now that we have labeled S1 and S2, here is a rendering of 2019-nCov Spike.
Overall, it has a similar appearance.
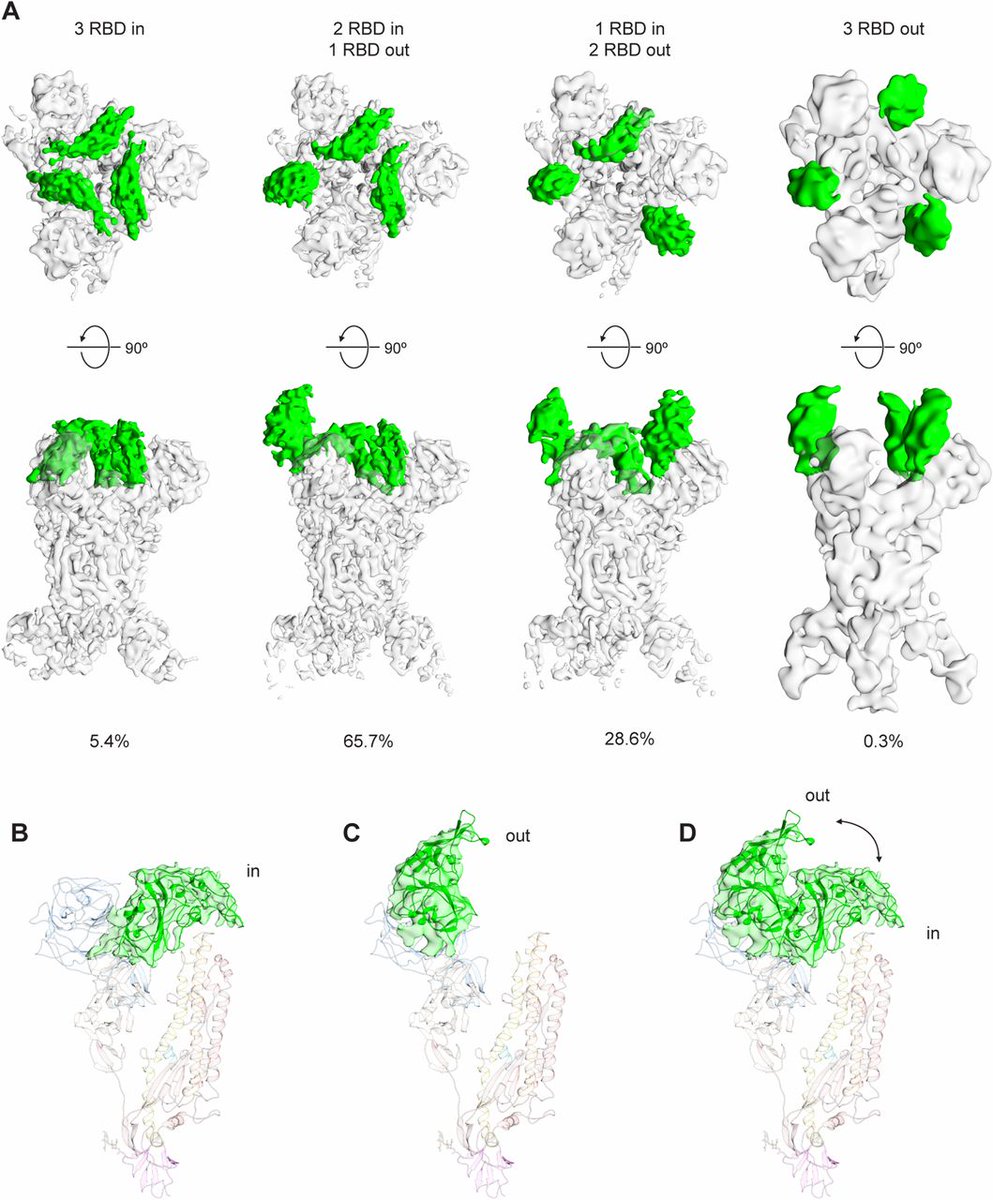
The receptor binding domains, RBD, protrude at the tip. Usually, one is open and two are closed.
https://www.nature.com/articles/nature17200?platform=oscar&draft=journal">https://www.nature.com/articles/...
Overall, it has a similar appearance.
The receptor binding domains, RBD, protrude at the tip. Usually, one is open and two are closed.
https://www.nature.com/articles/nature17200?platform=oscar&draft=journal">https://www.nature.com/articles/...
The appearance somewhat resembles a claw.
Here is what they look like in various folded and unfolded states of the MERS-CoV betacoronavirus, for instance:
Here is what they look like in various folded and unfolded states of the MERS-CoV betacoronavirus, for instance:
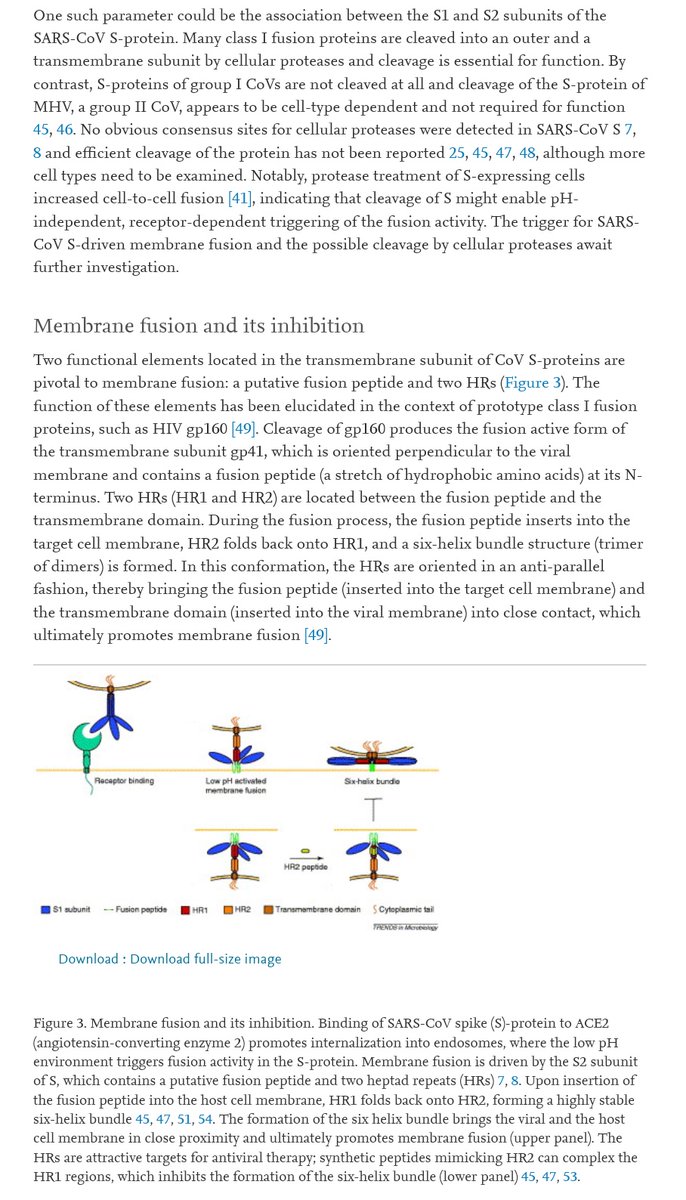
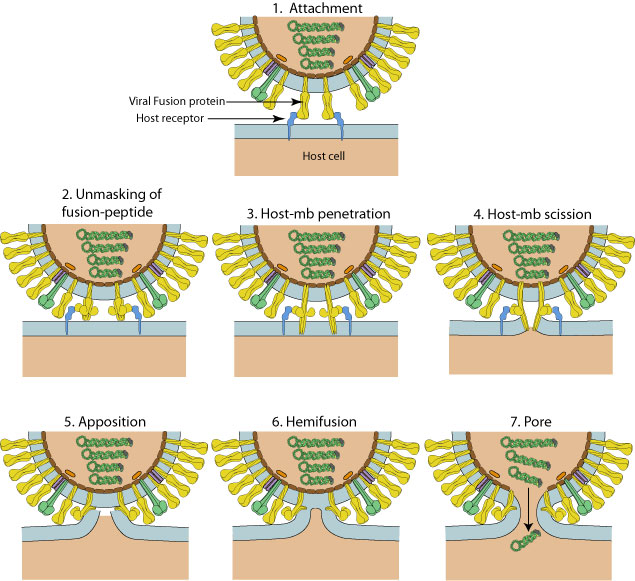
S1 binds, anchoring the virus to ACE2 via RBD.
Cleavage of S2 from S1 causes S2 to rotate forward, jamming the fusion protein FP through the cell membrane to form a second anchor.
Spike HR1 and HR2 domains pair up into 6-HB, pulling the viral membrane against the cell membrane.
Cleavage of S2 from S1 causes S2 to rotate forward, jamming the fusion protein FP through the cell membrane to form a second anchor.
Spike HR1 and HR2 domains pair up into 6-HB, pulling the viral membrane against the cell membrane.
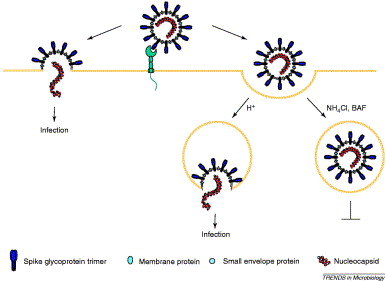
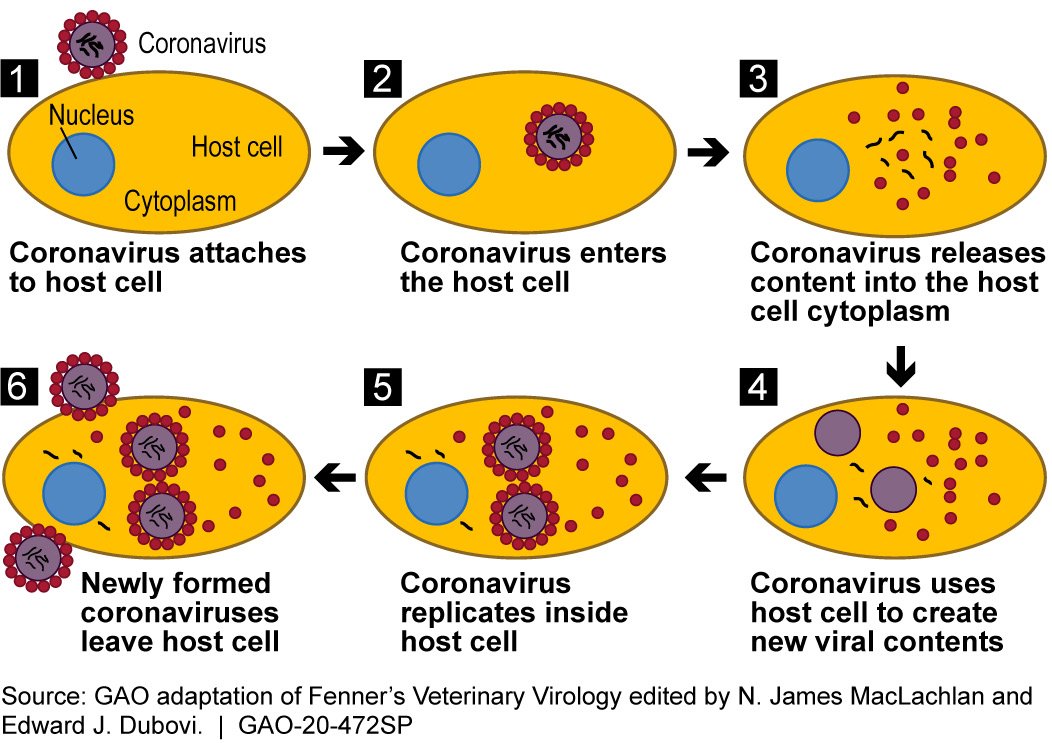
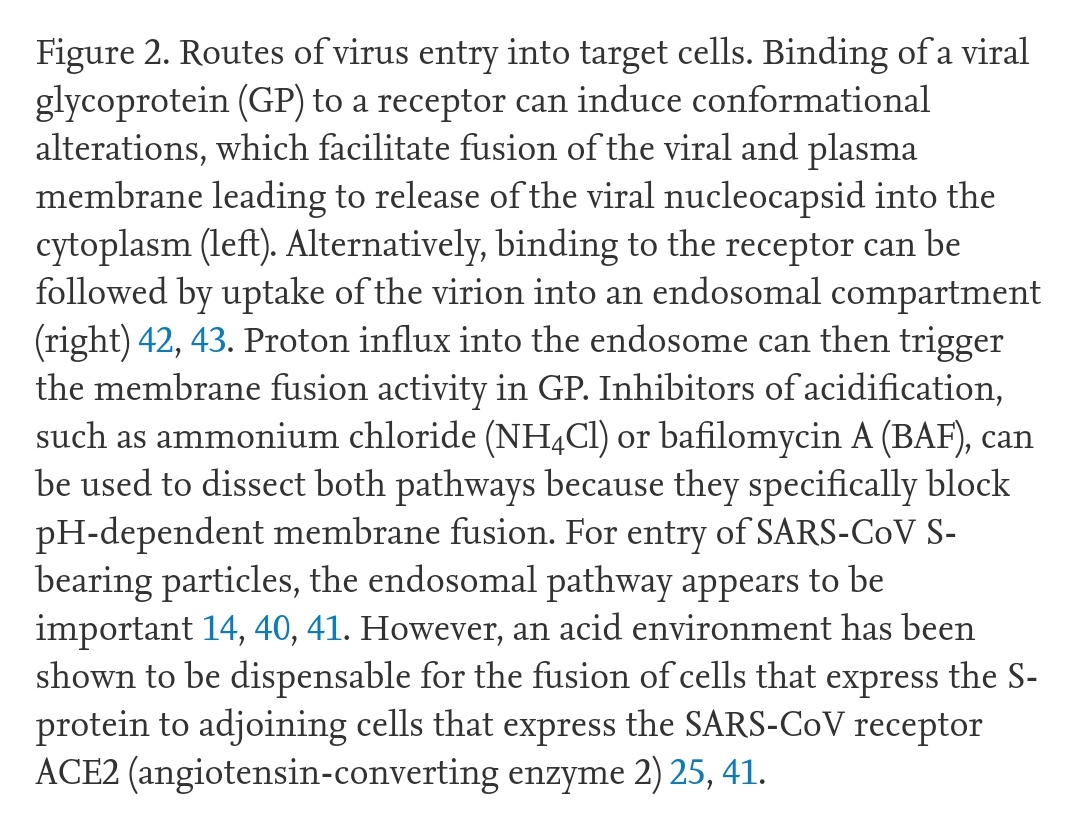
This process usually occurs within a vesicle formed from the cell membrane, containing the virus and allowing it into the cell. The vesicle forms after the virus binds to ACE-2.
https://www.sciencedirect.com/science/article/pii/S0966842X04001878">https://www.sciencedirect.com/science/a...
https://www.sciencedirect.com/science/article/pii/S0966842X04001878">https://www.sciencedirect.com/science/a...
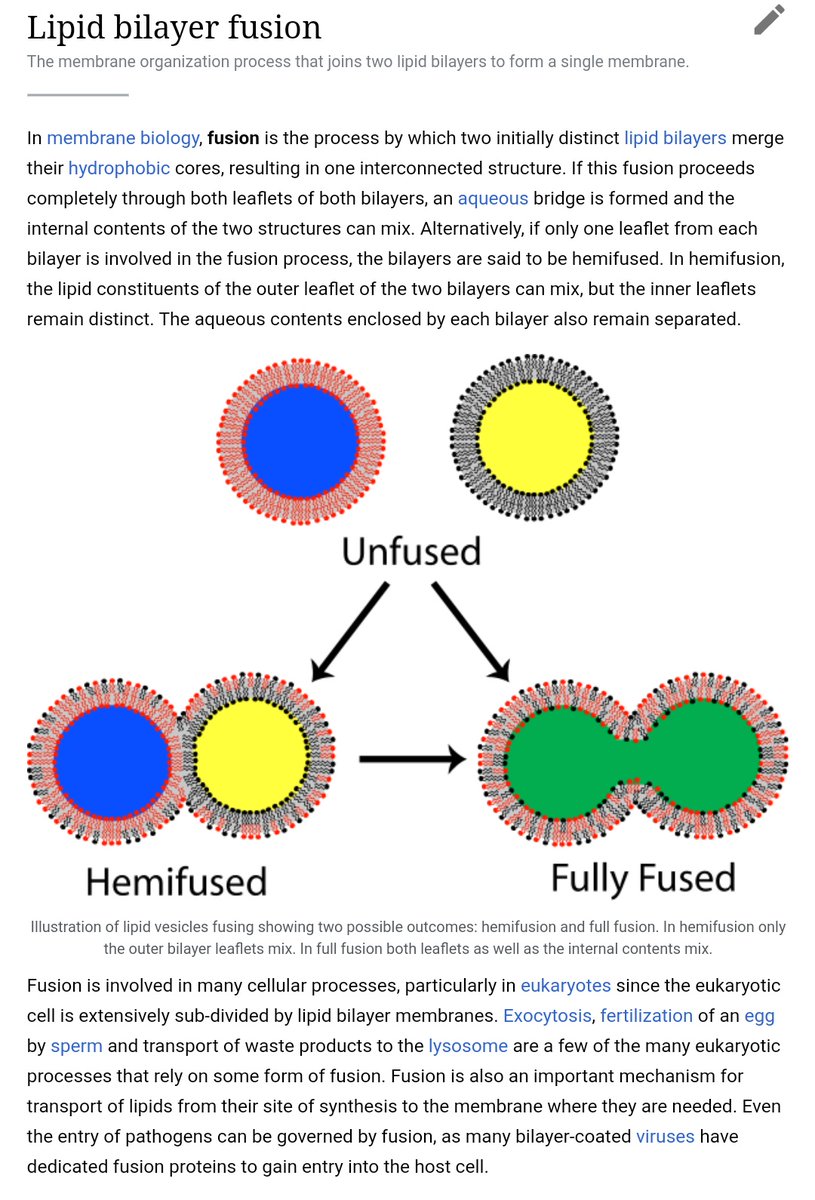
Here is an illustration of the stage at which the fusion protein is inserted through the cell membrane.
Once this second anchor is available, the trimeric structure flattens and pulls the two membranes together against it, resulting in their fusion and coalescence.
Once this second anchor is available, the trimeric structure flattens and pulls the two membranes together against it, resulting in their fusion and coalescence.
The virus everts itself at the location of membrane fusion, projecting its internal contents freely into the cell.
Note the dimerized & #39;opening& #39; process forming the transmembrane domain, just prior to eversion.
Note the dimerized & #39;opening& #39; process forming the transmembrane domain, just prior to eversion.
So, how is this relevant to treatment of COVID-19?
Chloroquine, for instance, appears to interfere with the fusion of the viral membrane with the internal surface of the vesicle (endosome) membrane, shown above. https://twitter.com/__ice9/status/1239404284086812672?s=19">https://twitter.com/__ice9/st...
Chloroquine, for instance, appears to interfere with the fusion of the viral membrane with the internal surface of the vesicle (endosome) membrane, shown above. https://twitter.com/__ice9/status/1239404284086812672?s=19">https://twitter.com/__ice9/st...
Arbidol (umifenovir) is also a viral membrane fusion inhibitor: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0015874">https://journals.plos.org/plosone/a...
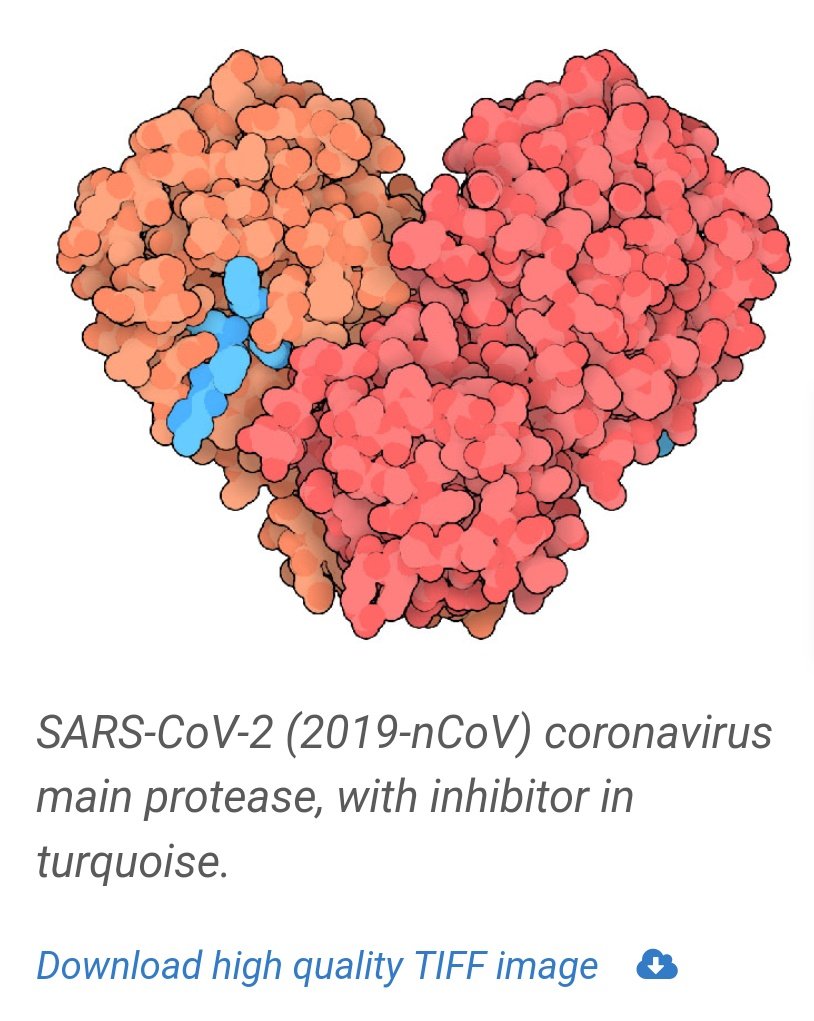
Lopinavir-ritonavir and nelfinavir, in contrast, are viral protease inhibitors.
They inhibit the protein-cleaving enzymes used by the virus to separate its individual proteins from the long chain protein produced when its RNA is read by a host ribosome.
https://pdb101.rcsb.org/motm/242 ">https://pdb101.rcsb.org/motm/242&...
They inhibit the protein-cleaving enzymes used by the virus to separate its individual proteins from the long chain protein produced when its RNA is read by a host ribosome.
https://pdb101.rcsb.org/motm/242 ">https://pdb101.rcsb.org/motm/242&...

 Read on Twitter
Read on Twitter