I& #39;m so excited to share our latest work where we show macrophages directly contributing collagen to scar formation in zebrafish and mouse hearts! https://www.nature.com/articles/s41467-019-14263-2">https://www.nature.com/articles/... A collaborative effort with fellows @GroupRiley @tomjcahill and @TSS_Lab @amykenyon3. And now a thread! 1/n
Billions of #cardiac muscle cells are lost during a #heartattack. The human heart cannot replenish these lost cells, so the default mechanism of repair is to form a permanent cardiac #scar. This leads to heart failure as the heart cannot pump as efficiently as before damage 2/n
#Zebrafish is able to fully #regenerate its #heart after damage by forming a temporary scar, at the same time that new cardiac #muscle cells are made. We wanted to understand whether we could modulate the mammalian #scar to become more like the zebrafish one, transient 3/n
To do so, we looked at 3 different post-injury environments: the #adultmouse heart, which behaves in a similar way to the #humanheart, the #neonatemouse heart, which can regenerate up to 7 days after birth, and the #zebrafish which can #regenerate the heart up to adulthood 4/n
We investigated whether #macrophages, cells of our #immunesystem which remove dead cells and help #repairing damaged tissue, behaved the same way in these 3 models. For zebrafish we generated nuclear #biotagging transgenic lines to look at macrophages’ active transcriptome 5/n
We were surprised: #macrophages were expressing molecules that form part of the #cardiac #scar, in particularly loads of #collagens, a key part of scar tissue. Quite striking  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😮" title="Gesicht mit offenem Mund" aria-label="Emoji: Gesicht mit offenem Mund"> because only cardiac myofibroblasts have been implicated in directly forming a scar in the heart 6/n
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😮" title="Gesicht mit offenem Mund" aria-label="Emoji: Gesicht mit offenem Mund"> because only cardiac myofibroblasts have been implicated in directly forming a scar in the heart 6/n
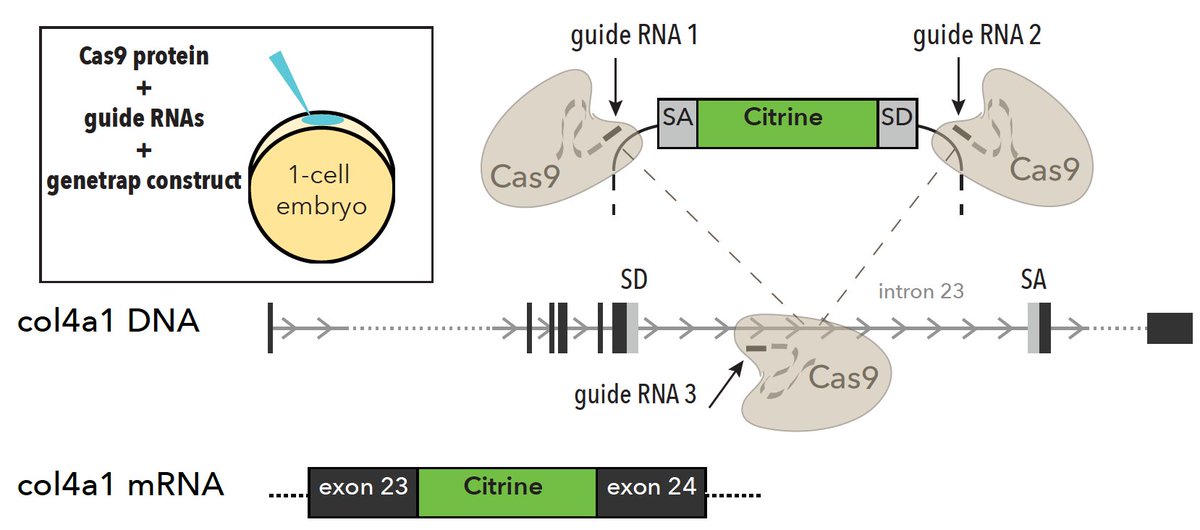
To understand whether #macrophages were in fact directly contributing to the scar, we endogenously #tagged #collagens with #GFP. In #zebrafish, we did this by combining homology independent repair-based genome editing #CRISPR w/ intron-based protein trapping: “trio” tagging! 7/n
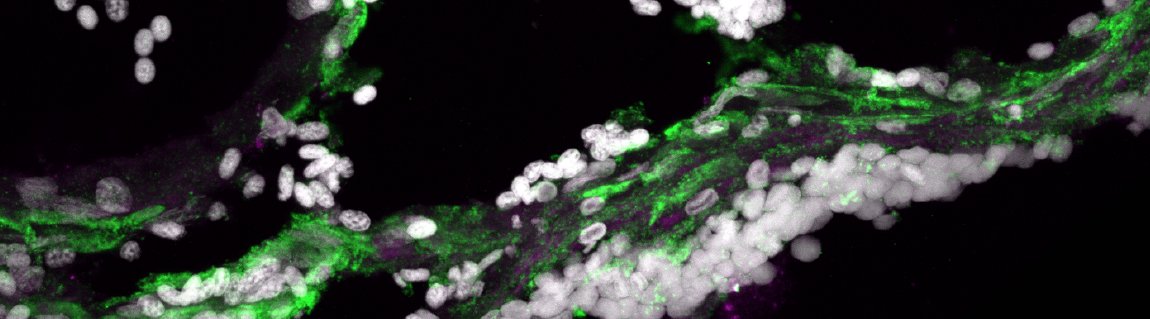
We then #transplanted the collagen-tagged #macrophages into both zebrafish and mouse injured hearts and looked 3 weeks later, a time point where the scar has been deposited. And Eureka! We were so surprised to see that part of the #ECM scar was green in its composition!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n
So #macrophages can upregulate #collagens, export them to the extracellular matrix #ECM and deposit into the #cardiac scar. The cool thing is that this new function is evolutionarily conserved since both zebrafish and mouse hearts had partly green scars! 9/n
Our work challenges the current #dogma that #myofibroblasts are the sole cells contributing to the cardiac scar. We hope that by showing that macrophages also produce collagen, a key part of scar tissue, new ways to enhance repair after a #heartattack can be found 10/n
But still many questions remain: why do #macrophages act as #collagen depositing cells during cardiac #woundhealing and #fibrosis? Are they playing a different role than #myofibroblasts? Much more curiosity-driven research needs to be done, but that is a different story  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht"> 11/n
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht"> 11/n
I cannot thank enough all the people involved in this work like twitter fellows @Joey_JMVieira @neutrinonstrike but the biggest shout-out goes to my wonderful #mentors Paul Riley and @saukaspengler for always being my allies! And of course @TheBHF for funding our research! 12/n
Check the full story for details here:
https://www.nature.com/articles/s41467-019-14263-2">https://www.nature.com/articles/...
@GroupRiley @TSS_Lab @DPAGPostdoc @MRC_WIMM @RDMOxford @OxfordMedSci #openaccess 13/n (fin) https://abs.twimg.com/emoji/v2/... draggable="false" alt="💔" title="Gebrochenes Herz" aria-label="Emoji: Gebrochenes Herz">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💔" title="Gebrochenes Herz" aria-label="Emoji: Gebrochenes Herz"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔜" title=""Soon" mit nach rechts zeigendem Pfeil darüber" aria-label="Emoji: "Soon" mit nach rechts zeigendem Pfeil darüber">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔜" title=""Soon" mit nach rechts zeigendem Pfeil darüber" aria-label="Emoji: "Soon" mit nach rechts zeigendem Pfeil darüber"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="💓" title="Schlagendes Herz" aria-label="Emoji: Schlagendes Herz">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💓" title="Schlagendes Herz" aria-label="Emoji: Schlagendes Herz">
https://www.nature.com/articles/s41467-019-14263-2">https://www.nature.com/articles/...
@GroupRiley @TSS_Lab @DPAGPostdoc @MRC_WIMM @RDMOxford @OxfordMedSci #openaccess 13/n (fin)

 Read on Twitter
Read on Twitter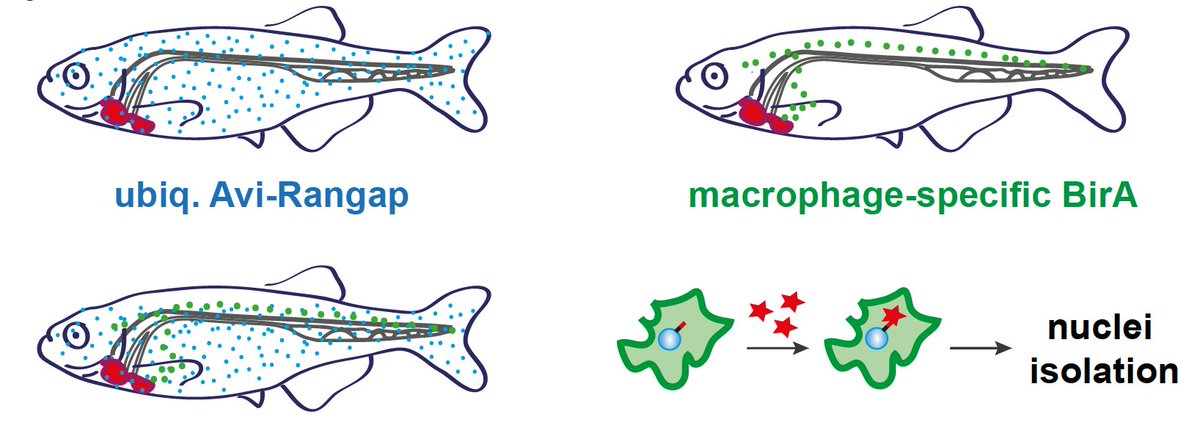
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n" title="We then #transplanted the collagen-tagged #macrophages into both zebrafish and mouse injured hearts and looked 3 weeks later, a time point where the scar has been deposited. And Eureka! We were so surprised to see that part of the #ECM scar was green in its composition! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n" class="img-responsive" style="max-width:100%;"/>
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n" title="We then #transplanted the collagen-tagged #macrophages into both zebrafish and mouse injured hearts and looked 3 weeks later, a time point where the scar has been deposited. And Eureka! We were so surprised to see that part of the #ECM scar was green in its composition! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥳" title="Partying face" aria-label="Emoji: Partying face"> 8/n" class="img-responsive" style="max-width:100%;"/>


