https://www.nejm.org/doi/pdf/10.1056/NEJMoa2115869?articleTools=true">https://www.nejm.org/doi/pdf/1...
/2 The 1st and most damaging issue that the trial was conducted in an area where IVM use was already exceptionally high. They **DID NOT EXCLUDE** for prior IVM use. Only "known hypersensitivity" to tested drugs. Case closed. This trial cannot deliver interpretable results.
/3a
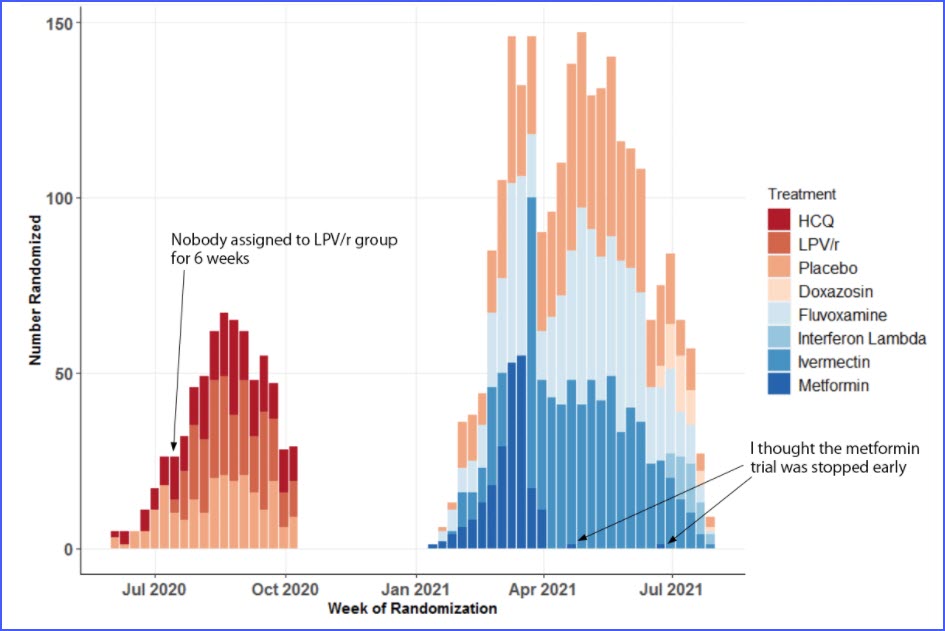
The randomization of the TOGETHER trial was **NOT RANDOM.**
The trial protocol states: "Participants are randomly assigned with equal allocation (...) Allocation of participants to treatment arms is uniform across all concurrent interventions as well as placebo."
The randomization of the TOGETHER trial was **NOT RANDOM.**
The trial protocol states: "Participants are randomly assigned with equal allocation (...) Allocation of participants to treatment arms is uniform across all concurrent interventions as well as placebo."
/3b
Did the TOGETHER randomization process equally and randomly assign patients to various arms in equal measures at equal times?
No. Case closed. This trial didn& #39;t even follow its own protocols.
Did the TOGETHER randomization process equally and randomly assign patients to various arms in equal measures at equal times?
No. Case closed. This trial didn& #39;t even follow its own protocols.

 Read on Twitter
Read on Twitter