Good morning and welcome to Day 2 of HDBuzz coverage of the CHDI HD Therapeutics conference! #HDTC2022
Chairing the third session of HD research talks is Dr. Michael Finley (CHDI) and Dr. William Martin (Janssen R&D, LLC) are chairing the third session of HD research talks, which will cover innovative approaches for HD therapeutics. #HDTC2022
Our first talk is from Dr. Beverly L Davidson from The Children’s Hospital of Philadelphia & University of Pennsylvania, who will discuss her work on improving gene therapies for HD. #HDTC2022
The Davidson lab works on making gene therapies to treat genetic illnesses like HD. She’s focused on what part of the huntingtin gene to target and how best to get drugs to the brain. Researchers want to make sure they’re doing this as efficiently as possible. #HDTC2022
As we learned yesterday, there are small toxic fragments of huntingtin that exist at the beginning of the code - exon1. The Davidson lab is focused on making sure this part of the huntingtin gene is targeted by the therapies they’re developing. #HDTC2022
The Davidson lab is working with CRISPR - this is a very precise tool which can edit specific letters in the DNA code. The lab aims to take advantage of unique genetic signatures, called SNPs (“snips”), to target the expanded huntingtin gene. #HDTC2022
Using this approach, researchers identify SNPs that are only on expanded huntingtin. This allows their potential therapeutics to specifically target only harmful huntingtin, leaving “normal” huntingtin alone. #HDTC2022
In a mouse model of HD, they showed that their CRISPR tool reduced the levels of huntingtin protein by about 50% - the magic number researchers think we need to lower huntingtin by to improve symptoms of HD. #HDTC2022
Next the Davidson lab focused on how to improve the way that these tools are delivered to cells. They want to make sure they’re effective and safe. #HDTC2022
The Davidson lab used a neat genetic trick to allow precise tuning to the expression level of the gene of interest, which you can think of like a dimmer switch. We previously wrote about this cool new tool here: https://en.hdbuzz.net/311 ">https://en.hdbuzz.net/311"...
This molecular dimmer switch could be really powerful for HD research - it could allow precise control of huntingtin levels, it gets directly to the right places in the brain, and leaves the body of the mouse quickly after they stop delivering it. #HDTC2022
The Davidson lab have now refined this tool for use in HD models and showed that they can fine tune huntingtin levels - the more drug they treat with, the more the dimmer switch is lowered. #HDTC2022
Moving forward, they’re focused on improving the way this CRISPR tool is delivered and testing it in other types of animals, including monkeys. #HDTC2022
They delivered this tool to the monkeys through a spinal injection and found that even very low doses reached lots of different areas in the brain, including those most affected by HD. #HDTC2022
Overall, the Davidson lab has developed an exciting new tool that targets only the expanded huntingtin copy and can reach many areas of the brain. This occurs even at low doses and can be precisely controlled. We’re excited to see where this goes next! #HDTC2022
Next up is Gene Yeo, from the University of California, San Diego, who will also be talking about CRISPR technology and testing genetic treatments in different animal models of HD. #HDTC2022
The Yeo lab is focused on understanding proteins that bind to the genetic message - RNA. They’re trying to target these RNA-binding proteins to develop therapeutics. #HDTC2022
RNA-binding proteins (RBPs) can control expression of other genes. The Yeo lab wants to know where RBPs bind, and have developed tools that let them learn this in individual cells - wow! #HDTC2022
Many experiments look at changes in whole tissues, or samples created from many cells. Looking at individual cells lets researchers zoom in on subtle but potentially important changes. #HDTC2022
A recent publication from the Yeo lab showed that they could use RPBs to bind to certain RNAs to “chew them up”. This would be great for destroying the huntingtin message to treat HD! #HDTC2022
Most recently, they have shown a decrease in the huntingtin message by delivering RBPs that specifically target CAG repeats. They can do this in multiple models, including human neurons created from stem cells. #HDTC2022
When the CAG repeats in the huntingtin message were destroyed, they were able to reverse some changes in cells caused by HD! One change they noticed was that expression of genes associated with brain cell health went back to normal. #HDTC2022
But they wanted to know what happens when they use this therapy in mice - does destroying the CAG repeats with their cool tool make the HD mice better? #HDTC2022
Yes! The mice did better on performance tests, had reduced huntingtin protein clumps, and improvements in brain structures seen by MRI. #HDTC2022
Also important, this genetic approach didn’t seem to affect other genes. This cool new tool still needs some validation but has lots of promise for many diseases - most excitingly, for HD! #HDTC2022
Our next speakers are Drs. Irina Antonijevic & Peter Bialek from Triplet Therapeutics. They’ll be discussing the SHIELD HD trial, a study that followed HD patients over time to try to find clinical differences and identify biomarkers. #HDTC2022
Triplet is researching therapies to combat the expansion of CAG repeats in brain cells over time, a process known as somatic instability or somatic expansion. This may be an important driver of when symptoms begin in people with HD. #HDTC2022
By looking at all the genetic data from individuals with HD, researchers identified changes in genes that control somatic instability that change the age that people with HD develop symptoms. One of those genes is called MSH3. #HDTC2022
While Triplet is developing a therapy that targets MSH3, they are also keen to better understand when best to treat patients and which patients would benefit most from the MSH3 targeting therapy. #HDTC2022
To better understand how CAG repeat expansion relates to HD symptoms, we need to follow people over time. SHIELD-HD is known as a natural history study - it does not involve a drug, but it is monitoring people with the HD gene who have very early symptoms. #HDTC2022
They followed HD patients for over 2 years and took various samples, including blood and CSF. They also analyzed the patients’ brains using MRI scans. #HDTC2022
They found that different regions of the brain, called the caudate and ventricles, changed their size over time during the 48 week period of the SHIELD-HD study. This is as we would expect as symptoms progress in people with HD. #HDTC2022
The study also looked at another measurement called the total motor score to see how this changed over time in people in the trial. As expected, this also decreased over time, and more so for patients at the later stages of HD. #HDTC2022
While these changes are expected in HD patients, the SHIELD-HD trial provides researchers with a comprehensive dataset that can be used to better make predictions about the course of HD. #HDTC2022
These types of datasets could help expedite finding the right type of clinical trial for patients based on where they are during their disease. #HDTC2022
Next, Triplet will share updates about their drug that targets the gene MSH3. They did experiments in monkeys to see how reducing MSH3 levels affected their CAG repeats. #HDTC2022
By lowering MSH3 by 50% in the monkeys, they found that somatic expansion was stopped! If this translates to HD patients, this might significantly delay the age at which patients start to develop symptoms. #HDTC2022
Triplet is also interested in measuring MSH3 levels to track HD disease progression and how well the treatment is working. But they ran into a challenge since it’s difficult to detect this gene in brain tissue. #HDTC2022
To get around this problem, the team at Triplet looked at expression of MSH3 in spinal fluid from participants with HD who were in the SHIELD-HD trial. They had to develop a very sensitive technique. #HDTC2022
They are continuing to experiment with different ways to collect samples from the spinal fluid and brain in monkeys, as well as testing the drug they are developing, called TTX-3360. #HDTC2022
They looked at levels of MSH3 in the CSF of patients at various disease stages. They found no difference in these levels between individuals without HD and those with HD who had no symptoms or were very early in their disease. #HDTC2022
This finding is important because it gives researchers at Triplet a baseline reading of MSH3 to follow for when they move TTX-3360 to a Phase 1 clinical trial and look to see how the levels of MSH3 change with treatment. #HDTC2022
Observational trials like SHIELD-HD not only collect lots of valuable data from HD patients over time, but they also allow researchers to develop new potential treatments like those described by Triplet today. Cool stuff! #HDTC2022
Time for a break! We’ll be back shortly for the rest of this mornings presentations. Stay tuned! #HDTC2022
Next up is Dr. Beth Stevens from Boston Children’s Hospital and the Broad Institute, who will be talking about her research that could provide insight for moving treatments toward the clinic. #HDTC2022
Dr. Stevens studies yet another specialized brain cell, called microglia, which act as the immune system of the brain, protecting it from invaders, and helping clean up debris left over from damaged brain cells. #HDTC2022
Microglia are tiny (thus the “micro”), and make up about only about 10% of the cells of the brain. But when they encounter damage, or invading bacteria, they get activated and go to work cleaning up the mess. #HDTC2022
This activation of these key helper cells is normally a good thing for the brain, but in a range of diseases - including HD - it has long been thought that they might be a little too active. Stevens is a world expert on the role of microglia in health and disease. #HDTC2022
Stevens has shown that one of the roles of microglia in the brain is to eat up synapses - the bulb-like links between communicating brain cells called neurons. Synapses are good, but need to be cleared to to encode new information into the brain. #HDTC2022
There’s a cell-to-cell communication system called the “complement system” that tells microglia to eat, or not to eat, a given synapse or cell. Years ago, Stevens’ team discovered that this complement system is used by microglia to decide which brain bits to digest. #HDTC2022
In many brain diseases - including HD - this complement system becomes over-active, eating bits clearly labeled with a “don’t eat me” signal for the complement system. #HDTC2022
The team is interested in understanding whether the complement system plays a key role in the loss of synapses known to happen in HD. #HDTC2022
They’ve developed very sophisticated microscope tricks to identify specific populations of synapses in brain regions impacted by HD. In HD mice, there’s a very specific pattern of synapse loss that worsens during aging. Similar changes are seen in HD patient brains. #HDTC2022
As they’d seen in other diseases, these same vulnerable synapses were decorated with “eat me” signals for the complement system. That suggests that microglia in HD might help remove these critical synapses from the brain, potentially contributing to disease progression. #HDTC2022
In brains donated by HD patients, Stevens’ team found clear evidence of angry, activated, microglia. They then turned back to mice, where they can manipulate this system to see what role it plays in disease progression. #HDTC2022
A company - Annexon Biosciences - has developed a drug that blocks complement activation. This allows us to ask whether blocking this hyper-active “eat me” activity contributes to the development of HD-like symptoms in HD model mice. #HDTC2022
Treating HD mice with this drug did what it was supposed to do - it reduced the “eat me” label from being placed onto critical brain regions. This allows us to ask whether this synapse removal is good or bad in diseases like HD. #HDTC2022
Using another approach - a genetic change to the mice to fully block the complement system - the team is studying the relationship between complement activation and symptoms. Excitingly, they see protection from some HD-like symptoms in HD model mice. #HDTC2022
But what about HD patients, do similar things happen in the brains of real patients? Using @clarity, the team was able to get access to cerebrospinal fluid from HD patients. This fluid, which bathes the brain, can be a non-invasive way to sample brain proteins. #HDTC2022
Consistent with their predictions, there were clear signs of increased activation of the complement system in the spinal fluid from HD patients. A small human study in HD patients is being conducted currently by Anexon. #HDTC2022
Very cool to see how seemingly very basic biological studies can be quickly translated to trials in HD patients! #HDTC2022
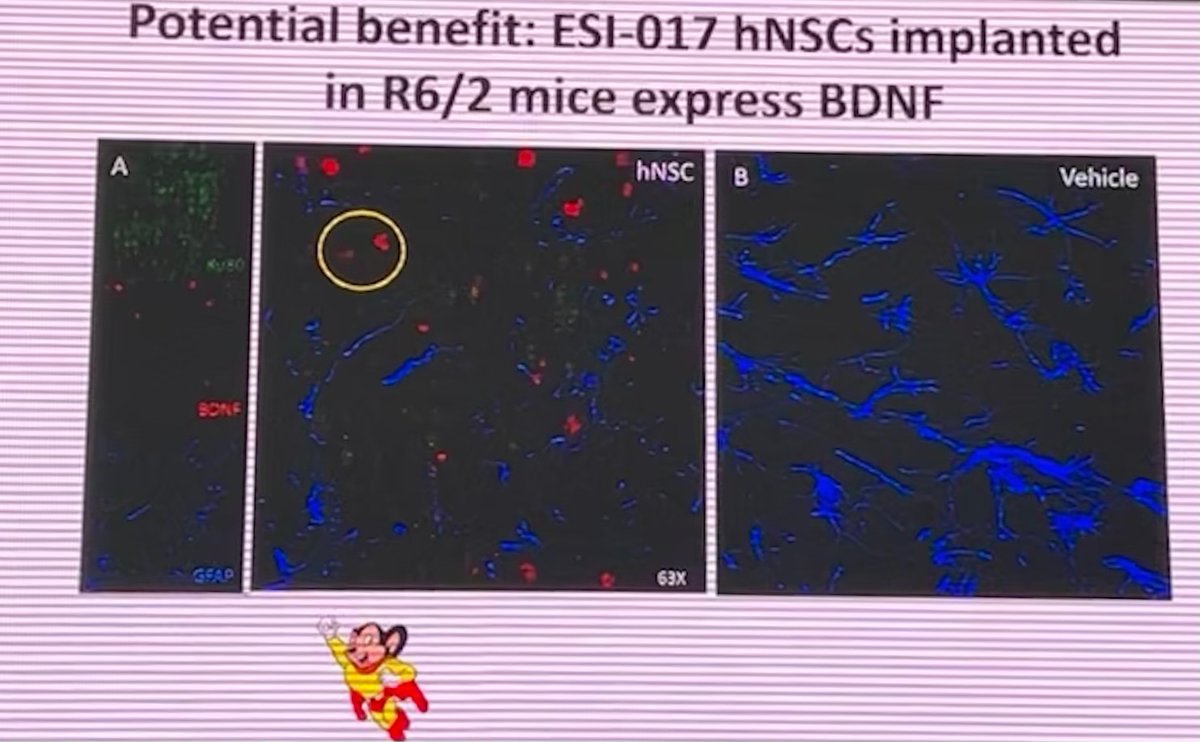
Dr. Leslie Thompson, from UC Irvine, is up next. Thompson has been a long-time leader in the field using stem cells to understand and treat HD. Stem cells are special cells that can be coaxed to become other cell types, including the vulnerable brain cells in HD. #HDTC2022
Historically, stem cells were isolated from embryos, but more recently researchers learned to coax regular cells from adults to become stem cells. These “induced pluripotent stem cells” are an amazing tool, allowing researchers to generate real brain cells in the lab #HDTC2022
Dr. Thompson represents a large consortium - called Stem Cells for HD (SC4HD) - who are coordinating efforts to develop potential cell-replacement treatments for HD. #HDTC2022
They’ve carried out huge studies to develop stem cell lines as a potential source for transplant studies into people with HD. Cells are complicated! The team has carried out a huge amount of standardization to make a very well-characterized source of donor cells. #HDTC2022
They’re using these human stem cell lines in experiments in HD mouse models to see whether transplanting cells into the brain improves HD-like symptoms in mice. Excitingly, transplantation of human stem cells leads to significant improvements. #HDTC2022
This is a proof of concept to show that implanting stem cells can lead to some improvements in HD-relevant symptoms in mice. Understanding the underpinnings of these improvements might allow the team to predict what symptoms to go after in HD patients #HDTC2022

 Read on Twitter
Read on Twitter