This is my first Thread/extensive research. Feel free to point anything wrong. Only for Educational Purpose.
Disc: Invested and biased. Invest at your own risk.
CONTENTS: -
1. Value Chain
2. Business Segments
3. Segmental Information.
1/x
4. Recent Acquisitions
5. Business Structure
6. Plants
7. Capex
8. Growth
9. My Thesis
10. Anti-Thesis
11. Moat
2/x
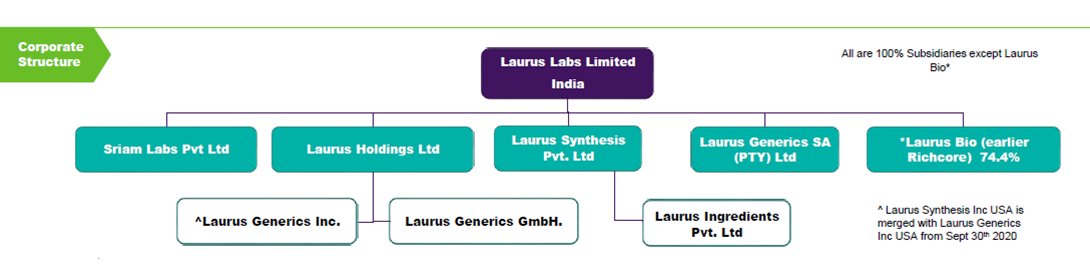
5. Business Structure
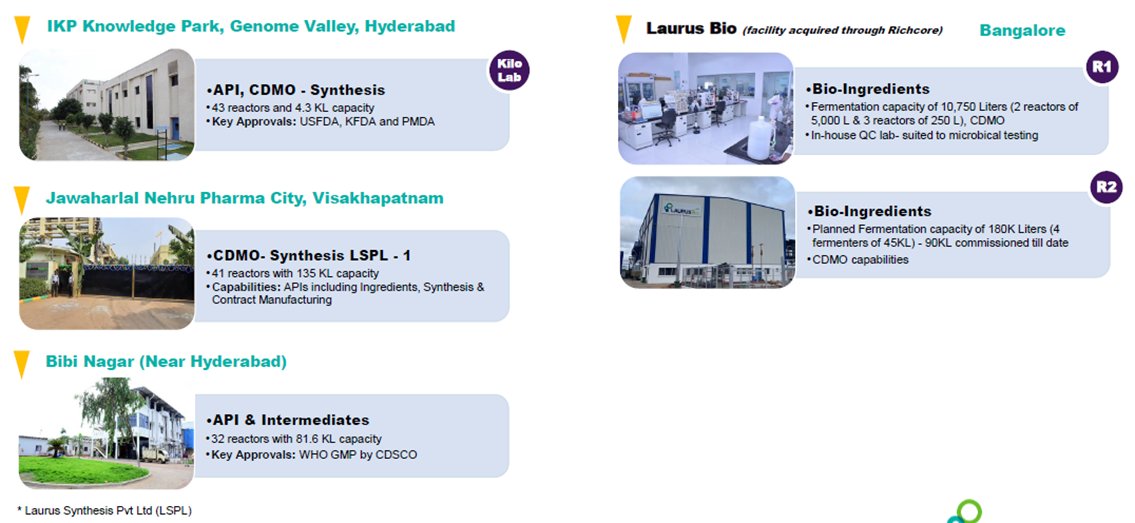
6. Plants
7. Capex
8. Growth
9. My Thesis
10. Anti-Thesis
11. Moat
2/x
• Today, we are among the world’s leading manufacturers of active pharmaceutical ingredients (API) for anti-retroviral (ARV), oncology, cardiovascular, antidiabetics, anti-asthma, and gastroenterology.
• Develop and manufacture oral solid formulations
3/x
• Develop and manufacture oral solid formulations
3/x
• R&D team comprises of 750+ scientists (~16% of total employee strength) including over 60 PhDs
• Also provide contract research and manufacturing services (CRAMS)
• Produce specialty ingredients for nutraceuticals, dietary supplements and cosmeceuticals
4/x
• Also provide contract research and manufacturing services (CRAMS)
• Produce specialty ingredients for nutraceuticals, dietary supplements and cosmeceuticals
4/x
VALUE CHAIN
From making just API’s (Active Substance), they now make all Advanced Intermediates they use and make formulations of the api’s they produce in the value chain.
(* shows backward & forward integration)
5/x
From making just API’s (Active Substance), they now make all Advanced Intermediates they use and make formulations of the api’s they produce in the value chain.
(* shows backward & forward integration)
5/x
Business Segments
• Laurus Generics - API - Development, manufacture and sale of APIs and advanced
intermediates
• Laurus Generics – Finished Dosage Form (FDF) - Development and manufacture of oral solid formulations from the api’s they make
6/x
• Laurus Generics - API - Development, manufacture and sale of APIs and advanced
intermediates
• Laurus Generics – Finished Dosage Form (FDF) - Development and manufacture of oral solid formulations from the api’s they make
6/x
• Laurus Synthesis - Key starting materials, intermediates and APIs for New Chemical Entities (NCEs)
• Laurus Bio – Recombinant products - animal origin free products for safer and viral free bio manufacturing
Quick update : now only 3 : Generics(API,FDF) , Synthesis, Bio
7/x
• Laurus Bio – Recombinant products - animal origin free products for safer and viral free bio manufacturing
Quick update : now only 3 : Generics(API,FDF) , Synthesis, Bio
7/x
Segmental Information
1. Laurus Generics – API
ARV API: Anti-Retroviral used in the treatment of HIV-AIDS. This is a 3yr tender driven business. When 1st line of drugs fails, then 2nd line of treatment drugs are given.
(Growth will be at max 5-8%)
8/x
(source: soicfinance)
1. Laurus Generics – API
ARV API: Anti-Retroviral used in the treatment of HIV-AIDS. This is a 3yr tender driven business. When 1st line of drugs fails, then 2nd line of treatment drugs are given.
(Growth will be at max 5-8%)
8/x
(source: soicfinance)
• Hepatitis C: Approved for Tenofovir Alafenamide used in the treatment of chronic hepatitis B virus infection with compensated liver disease.
• Non-ARV:
o anti-diabetic: 2 products already validated
o Cardiovascular: conditions affecting the heart or blood vessels
9/x
• Non-ARV:
o anti-diabetic: 2 products already validated
o Cardiovascular: conditions affecting the heart or blood vessels
9/x
o anti-asthma: Breathing problems
o gastroenterology: focused on the digestive system and its disorders
o Proton Pump Inhibitors (PPIs): reduction of stomach acid production
10/x
o gastroenterology: focused on the digestive system and its disorders
o Proton Pump Inhibitors (PPIs): reduction of stomach acid production
10/x
2. Laurus Generics – Formulations
Development and manufacturing of oral solid formulations for low and middle-income countries (LMIC), North America and European Union (EU) markets
Formulations are made using API’s they make. (Types of formulations are same as API’s)
11/x
Development and manufacturing of oral solid formulations for low and middle-income countries (LMIC), North America and European Union (EU) markets
Formulations are made using API’s they make. (Types of formulations are same as API’s)
11/x
3. Laurus Synthesis (CDMO)
• Steroids and hormone manufacturing capability
• specialty ingredients for use in nutraceuticals, dietary supplements and cosmeceutical products.
4. Laurus BIO
Acquired 72.55% stake in Richcore Lifesciences and renamed to Laurus Bio.
12/x
• Steroids and hormone manufacturing capability
• specialty ingredients for use in nutraceuticals, dietary supplements and cosmeceutical products.
4. Laurus BIO
Acquired 72.55% stake in Richcore Lifesciences and renamed to Laurus Bio.
12/x
Entering high-growth areas of recombinant animal origin free products (nonanimal cultured meat)
Laurus Bio operates through three distinct revenue streams – biotech, enzymes and CDMO
13/x
Laurus Bio operates through three distinct revenue streams – biotech, enzymes and CDMO
13/x
Going ahead, the CDMO segment is likely to be a major contributor to growth as a major portion of the incremental capacities are towards this business.
( clear play on operating leverage hinted ) *biased
14/x
( clear play on operating leverage hinted ) *biased
14/x
Recent Acquisitions
[JAN 21] Acquired 72.55% in Richcore Lifesciences to enter Biologics. Renamed to Laurus Bio Private Limited.
[JAN 22] to acquire 26.62% stake in Immunoadoptive Cell Therapy Private Limited (lmmunoACT), an advanced cell and gene therapy company.
15/x
[JAN 21] Acquired 72.55% in Richcore Lifesciences to enter Biologics. Renamed to Laurus Bio Private Limited.
[JAN 22] to acquire 26.62% stake in Immunoadoptive Cell Therapy Private Limited (lmmunoACT), an advanced cell and gene therapy company.
15/x
Treatment for Immuno deceases and particularly for Cancer treatment.
This investment provides Laurus an access and entry into CAR-T therapy.
A very promising treatment option which has had great success in the western part of the world.
16/x
This investment provides Laurus an access and entry into CAR-T therapy.
A very promising treatment option which has had great success in the western part of the world.
16/x
In India, CAR-T therapy is not available, and this collaboration will help us in bringing this novel technology to the Indian patients at a very affordable pricing.
Must Read on this here : https://twitter.com/itsTarH/status/1461635789071151109?s=20
(">https://twitter.com/itsTarH/s... can be fully acquired in the coming years )
17/x
Must Read on this here : https://twitter.com/itsTarH/status/1461635789071151109?s=20
(">https://twitter.com/itsTarH/s... can be fully acquired in the coming years )
17/x
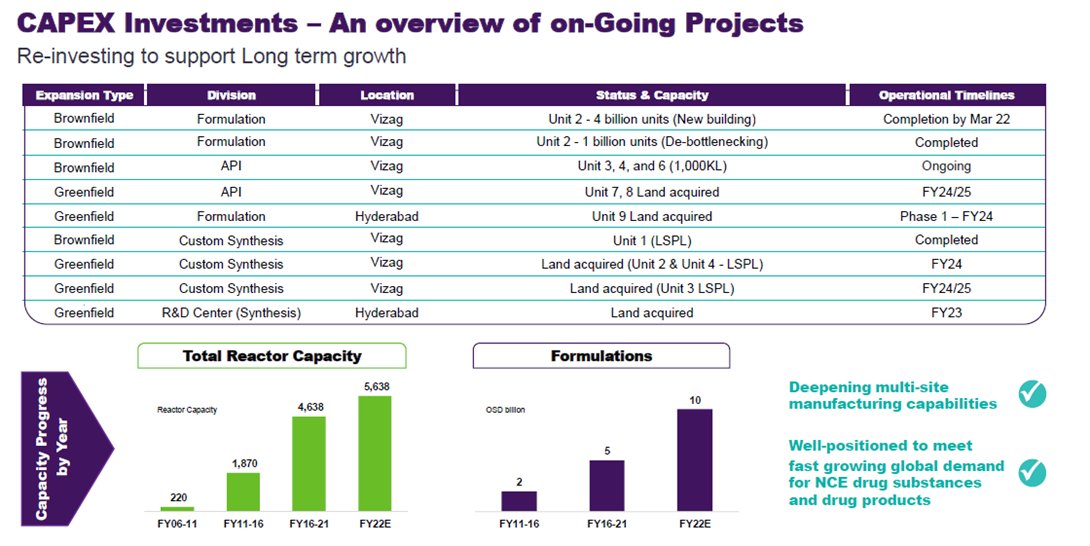
GROWTH via Capacity Expansion:
API’s: +21.56% increase when done(ongoing)
Formulations: +66.66% increase starting from FY23(Mar2022)
Synthesis: now 5535KL capacity. More coming in FY 2023,2024,2025.
Bio: +90KL fermentation capacity to the existing 90KL.
21/x
API’s: +21.56% increase when done(ongoing)
Formulations: +66.66% increase starting from FY23(Mar2022)
Synthesis: now 5535KL capacity. More coming in FY 2023,2024,2025.
Bio: +90KL fermentation capacity to the existing 90KL.
21/x
THESIS
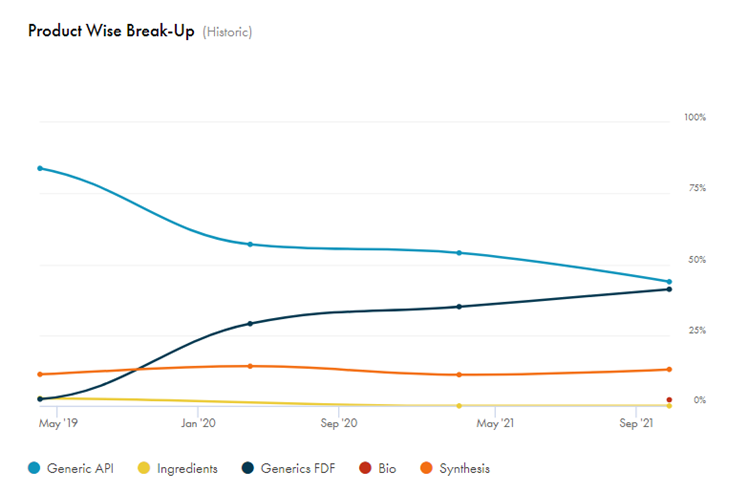
Walking the Talk & Excellent Capital Allocation: Revenue mix has improved from ARV to other segments and also shows excellent capital allocation of taking cashflow from a lumpy ARV api business into Non-ARV api’s, backward integration into intermediates
22/x
Walking the Talk & Excellent Capital Allocation: Revenue mix has improved from ARV to other segments and also shows excellent capital allocation of taking cashflow from a lumpy ARV api business into Non-ARV api’s, backward integration into intermediates
22/x
forward integration into formulations, synthesis(CDMO) and now Bio.
Laurus API & Formulations: Diversification from ARV api to Anti-diabetic, cardiovascular and others.
Formulations are now also going to be done for NON-ARV api’s (start of FY23).
23/x
Laurus API & Formulations: Diversification from ARV api to Anti-diabetic, cardiovascular and others.
Formulations are now also going to be done for NON-ARV api’s (start of FY23).
23/x
Laurus Synthesis: Developing capacities where once operating leverage kick’s-in, it’s a bomb. (fixed cost + lower incremental costs and exponential revenues)
Laurus Bio: sky is the limit. hinted for 1mil, then 3-4mil capacity. so, ~5x, then ~19x revenue from bio if done.
24/x
Laurus Bio: sky is the limit. hinted for 1mil, then 3-4mil capacity. so, ~5x, then ~19x revenue from bio if done.
24/x
Addition of high Potent Molecules from 2025: Maybe because they are currently working on it (HIGH PROBABLITY) but the existing drugs might go off patent in 2025.
Dr. Satyanarayana Chava: "These launches will happen after 2025."
25/x
Dr. Satyanarayana Chava: "These launches will happen after 2025."
25/x
Injectables: They currently only do oral solids. They used to doge injectables. But, in the Nov’21 concall, finally they said of entering into the segment.
26/x
26/x
Anti-Thesis
Raw Material: High Dependence on Raw Material imports from China.
Injectables: Not easy to get process expertise. Then comes approvals. Then comes manufacturing part.
Regulatory Risks: The major risk in the company is any USFDA export ban on the facilities.
27/x
Raw Material: High Dependence on Raw Material imports from China.
Injectables: Not easy to get process expertise. Then comes approvals. Then comes manufacturing part.
Regulatory Risks: The major risk in the company is any USFDA export ban on the facilities.
27/x
Wipeout of ARV api’s: Recently there has been a clearance of a new injectable drug, that can replace ARV drugs consumed in oral solids way (pills, tablets) as it is 1/4th the cost and less dosage.
Must read: https://twitter.com/Dr_Midhun_cs/status/1463165201809825792
28/x">https://twitter.com/Dr_Midhun...
Must read: https://twitter.com/Dr_Midhun_cs/status/1463165201809825792
28/x">https://twitter.com/Dr_Midhun...
Also, ARV api is a tender driven business (3 years). If their customers don’t win bids, they might have some revenue fall.
High Freight Charges: The company had to suffer a bit in their earnings for the freight charges due to less availability of containers.
29/x
High Freight Charges: The company had to suffer a bit in their earnings for the freight charges due to less availability of containers.
29/x
So, we must have this in our mind if this thing comes again in future.
Laurus Bio unable to scale: Considering Bio a new stream, if this segment does not grow, things can go wrong.
Debt: Even tough the debt is ok considering the upcoming growth, (D/E = 0.53).
30/x
Laurus Bio unable to scale: Considering Bio a new stream, if this segment does not grow, things can go wrong.
Debt: Even tough the debt is ok considering the upcoming growth, (D/E = 0.53).
30/x
However, the upcoming greenfield capex in the 3-4 land acquired can be a pain point as well.
They weren’t looking to reduce debt as growth opportunity was very good.
Now suddenly, within 3-4 months, they say, debt reduction might happen in the next year.
31/x
They weren’t looking to reduce debt as growth opportunity was very good.
Now suddenly, within 3-4 months, they say, debt reduction might happen in the next year.
31/x
MOAT
• USFDA approvals & compliance is not easy
• Biologics is a high gestation & high investment area within the Pharma industry. Typical development times range in many years and take about 5+ years on average to really scale up and transition to commercial production
32/x
• USFDA approvals & compliance is not easy
• Biologics is a high gestation & high investment area within the Pharma industry. Typical development times range in many years and take about 5+ years on average to really scale up and transition to commercial production
32/x
(from, Tar’s tweet mentioned earlier)
• Economies of scale in ARV api. Replication in non-arv api expected.
• Backward and forward integrated which helps them absorb price hikes of solvents/KSM.
33/x
• Economies of scale in ARV api. Replication in non-arv api expected.
• Backward and forward integrated which helps them absorb price hikes of solvents/KSM.
33/x
Valuations, abb, ye aap log decide karlo, coz, neend aa ri ab. gd nt. Else, i& #39;ll do it tmrw.
But, it is a very long term bet.. the real story will start when cdmo starts picking up.. from FY24 and FY25.
NOTE : NOT AN INVESTMENT ADVICE. PLEASE CONSULT YOUR FINANCIAL ADVISOR.
But, it is a very long term bet.. the real story will start when cdmo starts picking up.. from FY24 and FY25.
NOTE : NOT AN INVESTMENT ADVICE. PLEASE CONSULT YOUR FINANCIAL ADVISOR.

 Read on Twitter
Read on Twitter

![Recent Acquisitions[JAN 21] Acquired 72.55% in Richcore Lifesciences to enter Biologics. Renamed to Laurus Bio Private Limited.[JAN 22] to acquire 26.62% stake in Immunoadoptive Cell Therapy Private Limited (lmmunoACT), an advanced cell and gene therapy company.15/x Recent Acquisitions[JAN 21] Acquired 72.55% in Richcore Lifesciences to enter Biologics. Renamed to Laurus Bio Private Limited.[JAN 22] to acquire 26.62% stake in Immunoadoptive Cell Therapy Private Limited (lmmunoACT), an advanced cell and gene therapy company.15/x](https://pbs.twimg.com/media/FKDX5BBaIAEsGmI.jpg)