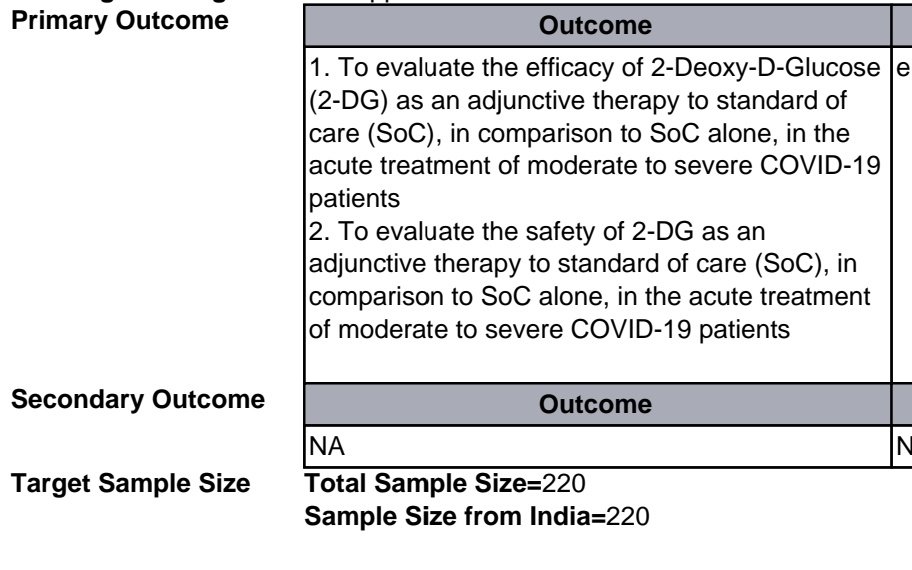
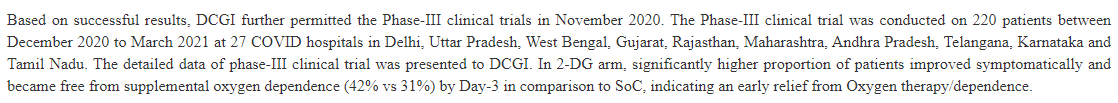So, I suspect most don& #39;t really get what the big deal is in not reporting the primary outcome of a trial before commencing the trial, so here& #39;s a short #tweetorial.. https://twitter.com/PriyankaPulla/status/1390993088886173697">https://twitter.com/PriyankaP...
So, let& #39;s say you& #39;re a competitor in a shooting contest, how do you measure your success? That& #39;s right - a target
Similarly, in clinical trials one starts with a target - aka primary endpoint. Generally this is the outcome of the trial you want to measure. These outcomes differ based on the kind of trial being planned. E.g. if we were planning a heart failure trial, some outcomes we might..
measure would be : improvement in LV function, improvement in symptom status, hospitalization, to just name a few. In Covid trials, these end points are determined based on the population we are studying, so for mild cases it might be time to recovery, or progression of disease.
For moderate to severe cases on the other hand, we tend to look at more serious endpoints like need for oxygen or mechanical ventilation or death. The outcome has to be relevant to the patient and it should easily measurable with limited scope for manipulation or bias.
Why else is identifying primary endpoints crucial in designing a trial? A: Because it also determines how many patients you have to recruit. This gives your trial adequate statistical power to confidently say that any difference you do eventually find is actually meaningful.
So what does it mean if I don& #39;t declare the primary endpoint and leave it nebulous and undefined?? "Efficacy" can mean many different things, to a sneaky investigator its meaning could be changed to suit whatever small difference your trial accidentally picked up by chance.
In fact, if I were a sneaky investigator, I would measure a hundred different things just in case one happens to show an incidental difference at the end of the trial, then point to that and say "See, I told you my drug works!!"
That is why endpoints HAVE to be "pre-specified"
That is why endpoints HAVE to be "pre-specified"
It would be like shooting a machine gun in the dark, switching on the lights later, then pointing at wherever my bullets landed, and saying "Yep, that& #39;s exactly what I meant to shoot". It& #39;s unethical and sneaky. It& #39;s why we have a database like CTRI in the first place
To prevent "gaming" of trials. Companies hate it when their trials end up negative, and that& #39;s why they certainly love it if they can hide the target from you, only to sneakily reveal it later with a flourish, and whaddaya know? There& #39;s an arrow there!!
So, this is why you should be suspicious of what DRDO and Dr. Reddy Labs are upto when they say 2-DG "works" especially given the soft end point they eventually reported in their press release
"Composite end point of symptom relief AND free from oxygen on Day 3" might just be the only significant thing they could find when they analysed their 100 measurements, and given this language it sure sounds like it. We won& #39;t know anyhow until they give us a preprint or publish.
Until then, take this press release with a HUGE truckload of salt. End of tweetorial

 Read on Twitter
Read on Twitter