The @WHO has authorized #Sinopharm vaccine for emergency use, which is great!
The hope is that the manufacturers will participate in #Covax program @CEPIvaccines as @mariangelasimao mentioned, which is especially important for LMICs and global fight with the pandemic (1/12) https://twitter.com/HNohynek/status/1390964483758170113">https://twitter.com/HNohynek/...
The hope is that the manufacturers will participate in #Covax program @CEPIvaccines as @mariangelasimao mentioned, which is especially important for LMICs and global fight with the pandemic (1/12) https://twitter.com/HNohynek/status/1390964483758170113">https://twitter.com/HNohynek/...
As I wrote before  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> and as the @WHO_Europe @WHO stated in the announcement yesterday, the efficacy of #Sinopharm vaccine in older adults > 60 yo couldn& #39;t be assesses.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> and as the @WHO_Europe @WHO stated in the announcement yesterday, the efficacy of #Sinopharm vaccine in older adults > 60 yo couldn& #39;t be assesses.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❓" title="Rotes Fragezeichen-Symbol" aria-label="Emoji: Rotes Fragezeichen-Symbol">In other words - we do not know how well this vulnerable group is protected
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❓" title="Rotes Fragezeichen-Symbol" aria-label="Emoji: Rotes Fragezeichen-Symbol">In other words - we do not know how well this vulnerable group is protected https://abs.twimg.com/emoji/v2/... draggable="false" alt="❓" title="Rotes Fragezeichen-Symbol" aria-label="Emoji: Rotes Fragezeichen-Symbol">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❓" title="Rotes Fragezeichen-Symbol" aria-label="Emoji: Rotes Fragezeichen-Symbol">
(2/12) https://twitter.com/marija_backovic/status/1389968933701173248?s=20">https://twitter.com/marija_ba...
(2/12) https://twitter.com/marija_backovic/status/1389968933701173248?s=20">https://twitter.com/marija_ba...
Moreover the @WHO recommends that countries using #Sinopharm vaccine conduct their own safety & effectiveness studies in the older adult group.
While I am a big supporter of @WHO @WHO_Europe and everything they do, this suggestion poses a fundamental problem. Here is why https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">(3/12)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">(3/12)
While I am a big supporter of @WHO @WHO_Europe and everything they do, this suggestion poses a fundamental problem. Here is why
Majority of the LMICs affected by #COVID19 already have their health systems stretched to the edge. They have problems getting enough vaccines, administering them, logistics, supplies, human resources etc. They& #39;re most likely already overworked and struggling (4/12).
So suggesting them to conduct their own safety and effectiveness studies is not reasonable and is not realistic. Some of these countries are so small that even if they had conducted the studies, the data sample would be too small for any conclusions to be made (take  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇲🇪" title="Flagge von Montenegro" aria-label="Emoji: Flagge von Montenegro">)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇲🇪" title="Flagge von Montenegro" aria-label="Emoji: Flagge von Montenegro">)
(5/12)
(5/12)
What I& #39;d suggest to @WHO_Europe @WHO @HNohynek @mariangelasimao is to ask the #Sinopharm officials for more data and more transparency. Why is this vaccine being authorized without the data being shared with the international scientific community is beyond me? (6/12)
I have looked carefully through the Evidence Assessment report prepared by the SAGE Working Group and #CNBG presentation.
The two documents can be found here:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> https://bit.ly/2Rgaakq
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> https://bit.ly/2Rgaakq
https://bit.ly/2Rgaakq&q... class="Emoji" style="height:16px;" src=" https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> https://bit.ly/3nQAKg3
(7/12)">https://bit.ly/3nQAKg3&q...
The two documents can be found here:
(7/12)">https://bit.ly/3nQAKg3&q...
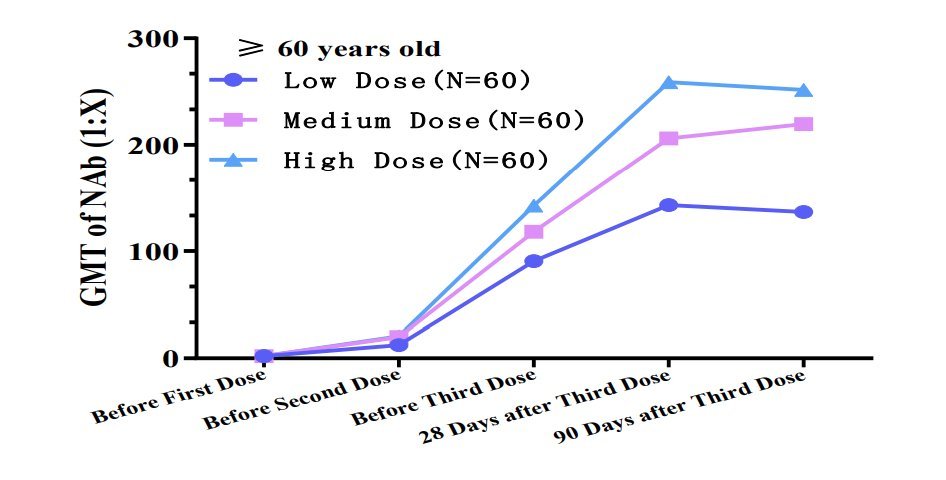
It& #39;s clear that trials were conducted in China showing adults >60 yo develop better response if they& #39;re given the vaccine in 3 dose regimen (G. Gao alluded to these tests). The spacing & amount of inactivated viruses per dose are not specified. But they must be known (8/12)
This is why I am asking @WHO officials to request #CNBG to release the info on:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🟣" title="Violetter Kreis" aria-label="Emoji: Violetter Kreis"> the details of 3 dose regimen for older adults
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🟣" title="Violetter Kreis" aria-label="Emoji: Violetter Kreis"> the details of 3 dose regimen for older adults
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🟣" title="Violetter Kreis" aria-label="Emoji: Violetter Kreis"> the antigen concentration in low/medium/high dose
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🟣" title="Violetter Kreis" aria-label="Emoji: Violetter Kreis"> the antigen concentration in low/medium/high dose
(9/12)
(9/12)
The SAGE Evidence Assessment was presented in part by Dr Kirsten Vannice who is a senior program officer @gatesfoundation, who is in turn in partnership with Beijing Bio-Institute Biological Products, subsidiary of CNBG and a manufacturer of #Sinopharm vaccine (10/12)
So there is a direct contact between @WHO and whoever presented the vaccine data on behalf of CNBG. Why wasn& #39;t there more scientific scrutiny and rigor when evaluating the data in particular related to the safety and efficacy in older adults? (11/12)
This would have be *so* helpful for the LMIC countries in adjusting their immunization protocols and protecting better the very vulnerable group of older adults. Not to mention securing on time extra doses if they need to administer 3 instead of 2...(12/12)
My final remark is that I hope #Sinopharm will make the vaccine affordable since it is one of the most expensive on the market at the moment (not helping the LMICs again)
Thanks for reading who managed to get to the end of this thread https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙂" title="Leicht lächelndes Gesicht" aria-label="Emoji: Leicht lächelndes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙂" title="Leicht lächelndes Gesicht" aria-label="Emoji: Leicht lächelndes Gesicht"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Gefaltete Hände" aria-label="Emoji: Gefaltete Hände"> https://www.nytimes.com/2021/03/11/world/hungary-sinopharm-covid.html">https://www.nytimes.com/2021/03/1...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Gefaltete Hände" aria-label="Emoji: Gefaltete Hände"> https://www.nytimes.com/2021/03/11/world/hungary-sinopharm-covid.html">https://www.nytimes.com/2021/03/1...
Thanks for reading who managed to get to the end of this thread

 Read on Twitter
Read on Twitter (3/12)" title="Moreover the @WHO recommends that countries using #Sinopharm vaccine conduct their own safety & effectiveness studies in the older adult group. While I am a big supporter of @WHO @WHO_Europe and everything they do, this suggestion poses a fundamental problem. Here is whyhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">(3/12)" class="img-responsive" style="max-width:100%;"/>
(3/12)" title="Moreover the @WHO recommends that countries using #Sinopharm vaccine conduct their own safety & effectiveness studies in the older adult group. While I am a big supporter of @WHO @WHO_Europe and everything they do, this suggestion poses a fundamental problem. Here is whyhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">(3/12)" class="img-responsive" style="max-width:100%;"/>
 https://bit.ly/2Rgaakq&q... class="Emoji" style="height:16px;" src=" " title="I have looked carefully through the Evidence Assessment report prepared by the SAGE Working Group and #CNBG presentation. The two documents can be found here:https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> https://bit.ly/2Rgaakq&q... class="Emoji" style="height:16px;" src=" " class="img-responsive" style="max-width:100%;"/>
https://bit.ly/2Rgaakq&q... class="Emoji" style="height:16px;" src=" " title="I have looked carefully through the Evidence Assessment report prepared by the SAGE Working Group and #CNBG presentation. The two documents can be found here:https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> https://bit.ly/2Rgaakq&q... class="Emoji" style="height:16px;" src=" " class="img-responsive" style="max-width:100%;"/>