It was good to discuss this with @HelenBranswell. Patents aren& #39;t a constraint to access. These things are:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Lack of capacity at originators to support more tech transfers;
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Lack of capacity at originators to support more tech transfers;
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Partners w/proven global vaccine manufacturing experience; and
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Partners w/proven global vaccine manufacturing experience; and
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Raw material/component shortages. 1/ https://twitter.com/HelenBranswell/status/1390274848392368133">https://twitter.com/HelenBran...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔹" title="Kleine blaue Raute" aria-label="Emoji: Kleine blaue Raute">Raw material/component shortages. 1/ https://twitter.com/HelenBranswell/status/1390274848392368133">https://twitter.com/HelenBran...
Scaling up vaccine manufacturing is *far* more complex than HIV medicines or other drugs. And the safety and benefit/risk calculation is completely different because vaccines are given to healthy people including children. 2/ https://twitter.com/rvenkayya/status/1356286204745015296?s=20">https://twitter.com/rvenkayya...
A national regulatory authority wouldn’t approve a “copied” vaccine unless there is robust “bridging” to the original process, testing and some clinical data (at a minimum). Same applies for @WHO for EUL (see companies approved to date). The bar is high, as it should be. 3/
All of this requires an extraordinary amount of time & effort to ensure the *exact* process (down to the specific raw materials, components, cell banks, virus seeds) has been transferred. This is *far* more complicated than HIV medicines or other drugs. 4/
In order to do this right, a team from the originator company has to be involved throughout the process. But that same small team is likely involved in all tech transfers and can only be stretched so thin before risks and quality issues emerge. We& #39;ve already seen this. 5/
It’s critical to have a partner with the necessary experience, expertise and infrastructure, one that has shown it can manufacture vaccines at global regulatory and quality standards. It would be a mistake to use an unproven manufacturer, their assurances notwithstanding. 6/
The worst outcome of an inadequate tech transfer, beyond an unapprovable product, is a quality or safety issue with vaccine produced at the new facility. That would certainly jeopardize confidence in the original vaccine and potentially others. 7/
No one should support manufacturing of vaccines that would not be authorized by the @WHO and/or stringent regulatory authorities. We cannot have a lower standard of quality and safety for certain countries, yet that is the potential outcome of some proposals on the table. 8/
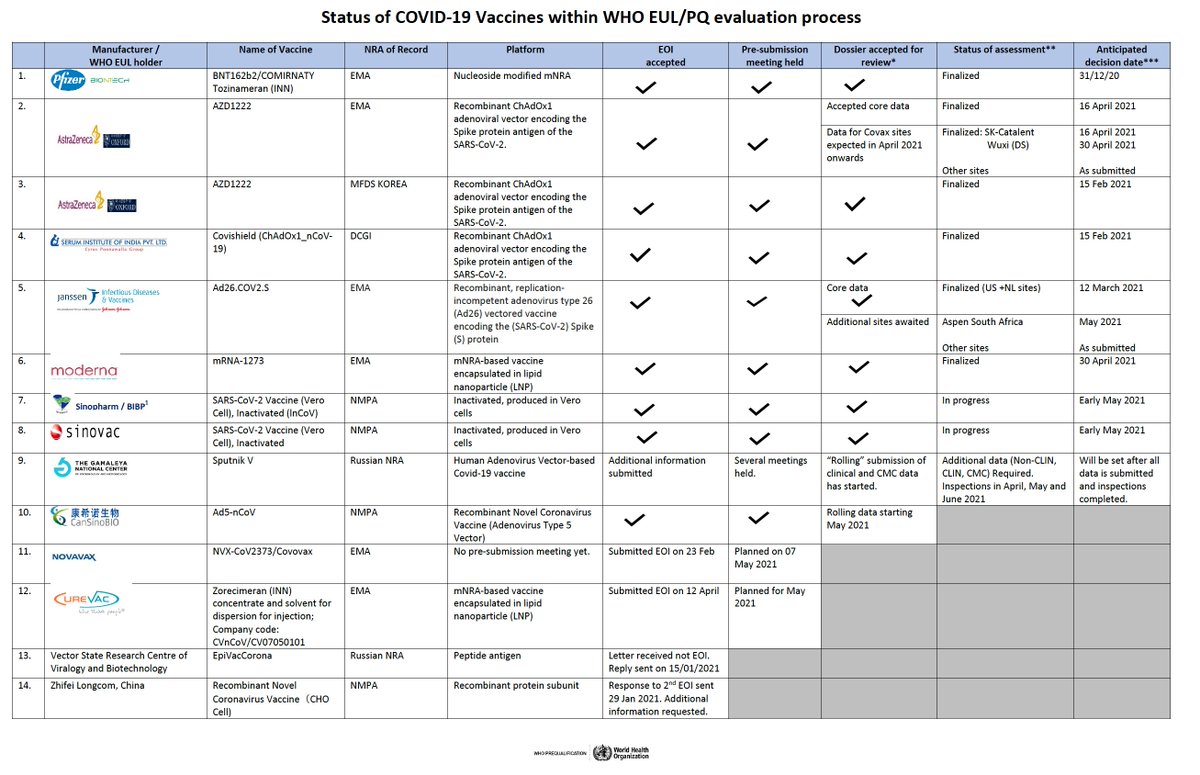
For reference, here is the status of @WHO EUL for vaccine manufacturers. Only a handful have been authorized so far, and they all have extensive vaccine manufacturing experience. 9/
https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_04May2021.pdf">https://extranet.who.int/pqweb/sit...
https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_04May2021.pdf">https://extranet.who.int/pqweb/sit...
What can we do? Expand capacity at facilities already making vaccines. Expand tech transfer teams. Augment regulatory capacity & expertise. Urgently address raw material and component constraints, which is a looming iceberg for the manufacturing of all biologics. 10/
Over the mid-term, we need to establish distributed vaccine manufacturing capacity, particularly in the African region. mRNA technologies present a gateway and leapfrog opportunity. We must start now but it will take time. 11/ https://twitter.com/rvenkayya/status/1385001589732257798?s=20">https://twitter.com/rvenkayya...
One immediate opportunity: reassess how vaccine is being allocated today. Is it appropriate for healthy young people in the US to be vaccinated, or for people to get a booster dose when healthcare workers and high-risk populations elsewhere haven’t been vaccinated? 12/
Many of us have raised concerns about inequitable access since early 2020, but there was little attention to the matter until the US secured enough supply for its population. It’s never too late to change direction, but we need to focus on things that will make a difference. 13/

 Read on Twitter
Read on Twitter