Curious about which antibodies work best for our best friend GFP in immunostaining? We want to know the answer too! So we quickly tested 12 anti-GFP antibodies we have in the lab and found our new favorites! Scroll down for how we tested them!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
Given so many candidates, we tried to narrow down our search first. We began with an endogenously eGFP-tagged DLG1 (PSD95 homolog in fly) line as our sample. As DLG is so abundant at NMJ, we were able to rule out some really bad ones that can’t even give a clear signal.
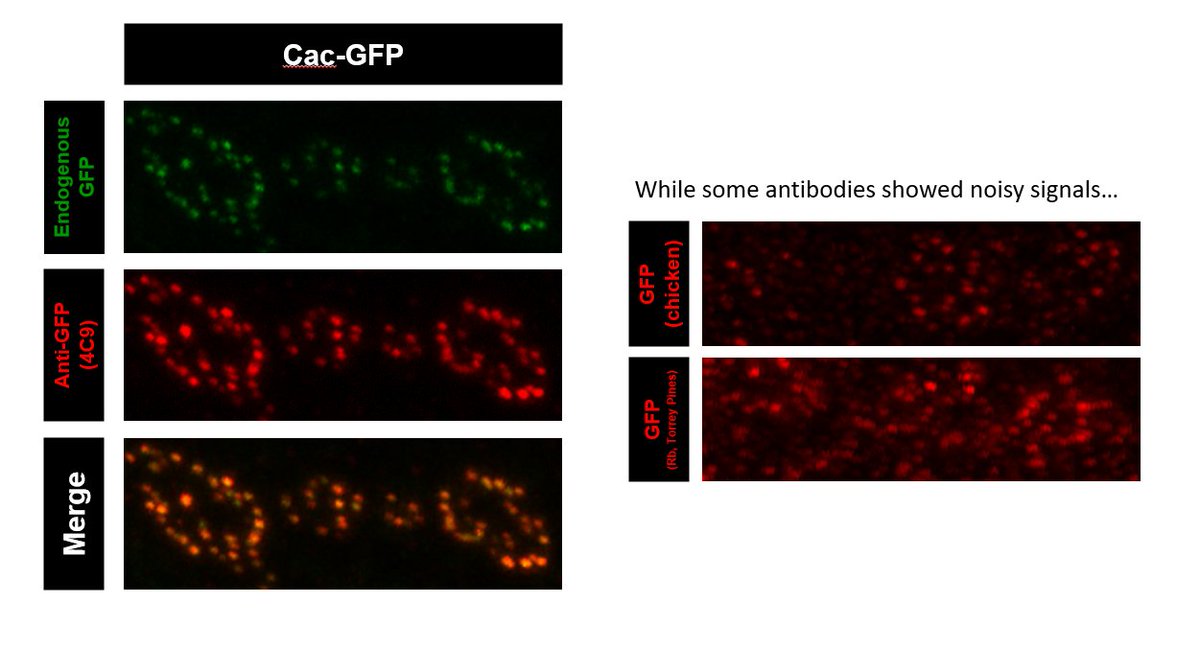
Here comes the real challenge! Second round we used flies tagging Cav2 Ca2+ channel Cac. Due to low protein abundance and puncta pattern of Cac, we hoped this test could further reveal really superb antibodies among the remaining candidates!
Results time! While some of the last round winners failed this time, a previously neglected mouse monoclonal turns out to be amazing! 4C9 antibody shows clear puncta structures, low background and great colocalization with endogenous GFP signals!
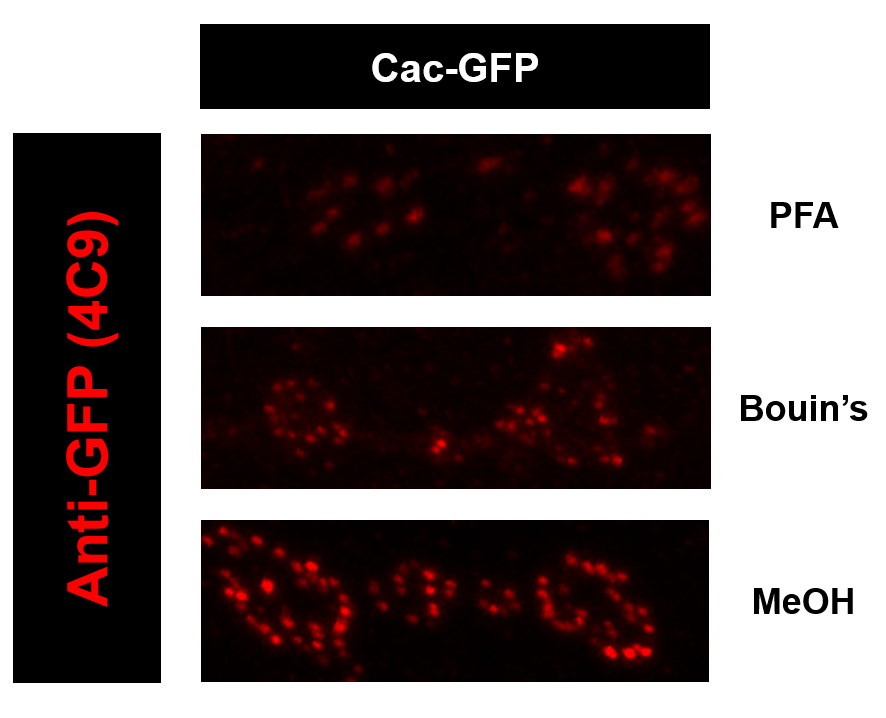
Meanwhile, we noticed that among the three common fixations (Bouin’s solution, PFA and MeOH), MeOH usually gives better performance than the other two.
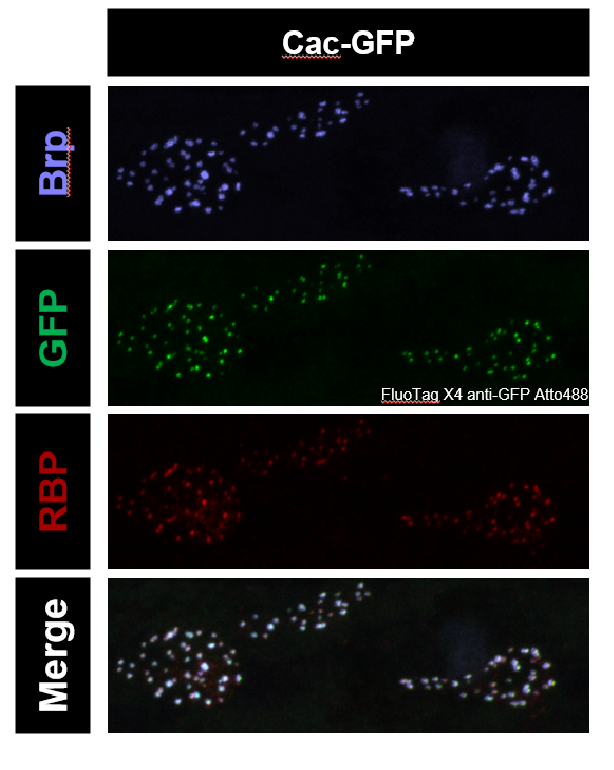
FluoTag-X4 nanobody Atto 488 also looks good! It made the dim endogenous signal remarkably visible. Look at how it beautifully co-localizes with other active zone components! We haven’t quantified how much the endogenous signal is enhanced at this point though.
Hope this is helpful! 4C9 has become our new favorite in our studies now. And we would love to hear your experience in using other anti-GFP antibodies, especially from host species other than mouse (we are still looking for great ones for co-staining!).
And a big shoutout for Dickman lab people @dion_synapse @YifuHan @ChunJChien (and also lab mates not yet on twitter)!! Glad we found these from the long dissection days https://abs.twimg.com/emoji/v2/... draggable="false" alt="😆" title="Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😆" title="Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen">

 Read on Twitter
Read on Twitter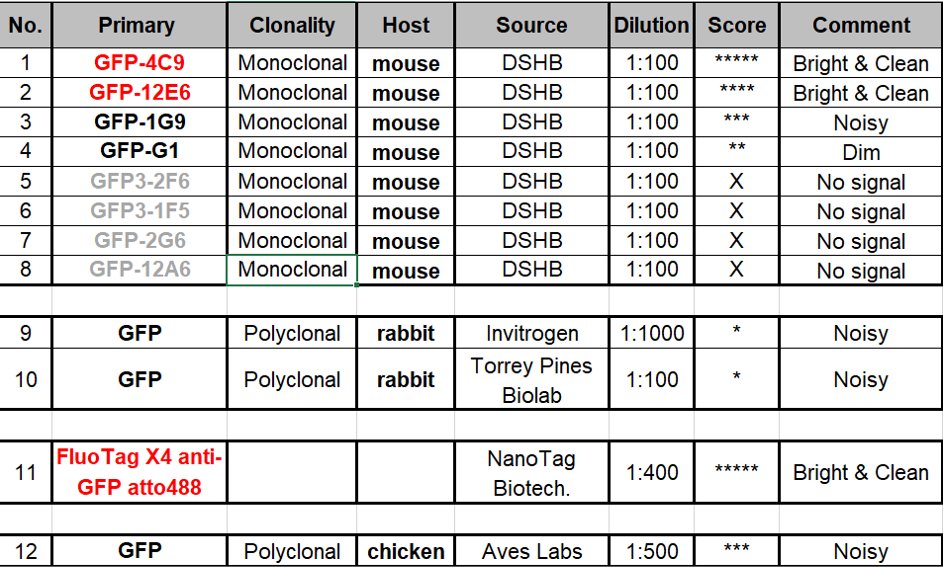 " title="Curious about which antibodies work best for our best friend GFP in immunostaining? We want to know the answer too! So we quickly tested 12 anti-GFP antibodies we have in the lab and found our new favorites! Scroll down for how we tested them! https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">" class="img-responsive" style="max-width:100%;"/>
" title="Curious about which antibodies work best for our best friend GFP in immunostaining? We want to know the answer too! So we quickly tested 12 anti-GFP antibodies we have in the lab and found our new favorites! Scroll down for how we tested them! https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">" class="img-responsive" style="max-width:100%;"/>