Inhaled budesonide. A thread.
There has been a lot of energy being given to budesonide in COVID-19, with some tweeterati referring to it as having "strong evidence".
Also, FPs/ED MDs would love a Rx that works!
It merits discussion.
Let me explain (long thread alert) ...
There has been a lot of energy being given to budesonide in COVID-19, with some tweeterati referring to it as having "strong evidence".
Also, FPs/ED MDs would love a Rx that works!
It merits discussion.
Let me explain (long thread alert) ...
There are 2 RCTs available on inhaled corticosteroids:
STOIC ( https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00160-0/fulltext#.YIqiWU0BDYU.twitter">https://www.thelancet.com/journals/... and https://clinicaltrials.gov/ct2/show/NCT04416399)">https://clinicaltrials.gov/ct2/show/... and PRINCIPLE ( https://www.medrxiv.org/content/10.1101/2021.04.10.21254672v1">https://www.medrxiv.org/content/1... and https://www.isrctn.com/ISRCTN86534580 )
I">https://www.isrctn.com/ISRCTN865... will start off by saying what we know about systemic corticosteroids in patients with COVID:
STOIC ( https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00160-0/fulltext#.YIqiWU0BDYU.twitter">https://www.thelancet.com/journals/... and https://clinicaltrials.gov/ct2/show/NCT04416399)">https://clinicaltrials.gov/ct2/show/... and PRINCIPLE ( https://www.medrxiv.org/content/10.1101/2021.04.10.21254672v1">https://www.medrxiv.org/content/1... and https://www.isrctn.com/ISRCTN86534580 )
I">https://www.isrctn.com/ISRCTN865... will start off by saying what we know about systemic corticosteroids in patients with COVID:
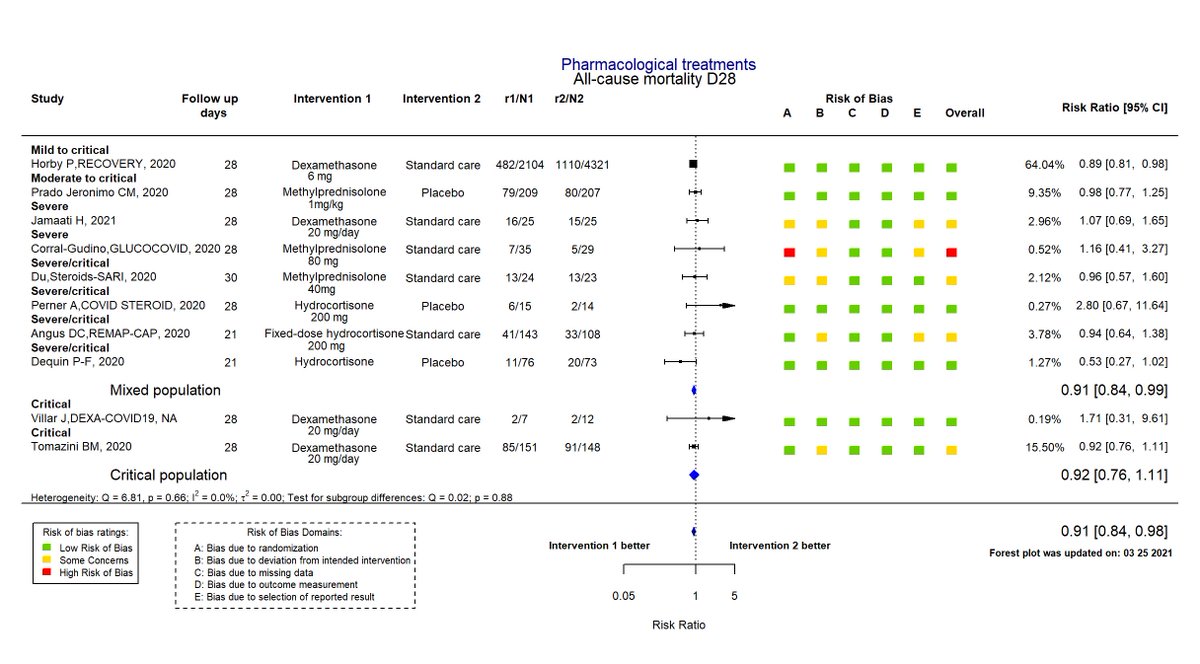
They likely work and save lives if patients need supplemental O2 or supported ventilation. The strongest evidence comes from the massive RECOVERY trial (N=6435, https://www.nejm.org/doi/full/10.1056/NEJMoa2021436).">https://www.nejm.org/doi/full/... Importantly, dexamethasone appeared potentially harmful for patients not requiring O2.
I& #39;m always reluctant to invoke mechanistic approaches—because they are so often wrong—but we know that early in disease virus is main player and, as disease progresses, inflammation is the problem.
The results of antiviral & anti-inflammatory studies are consistent w/this.
The results of antiviral & anti-inflammatory studies are consistent w/this.
But maybe systemic steroids aren& #39;t beneficial early on because systemic toxicity outweighs any benefit. I can buy this, which is why we need RCTs to evaluate this.
These studies were also prompted by observation early on that pts w/COPD, asthma underrepresented in cases.
STOIC:
These studies were also prompted by observation early on that pts w/COPD, asthma underrepresented in cases.
STOIC:
Adults in Oxfordshire, UK
Strat by age, sex, comorbid
Phase 2
New-onset (<8d) COVID symptoms
Budesonide inh 400mcg bid up to 28d vs. usual care (i.e. unblinded, advised to take antipyretics for fever and honey for cough)
n=146, mean age 44.5
1o outcome: 28d urgent care/ED visit
Strat by age, sex, comorbid
Phase 2
New-onset (<8d) COVID symptoms
Budesonide inh 400mcg bid up to 28d vs. usual care (i.e. unblinded, advised to take antipyretics for fever and honey for cough)
n=146, mean age 44.5
1o outcome: 28d urgent care/ED visit
ITT Results: 11 (15%) in usual care, 2 (3%) budesonide group.
Sounds great, right? NNT=8!
Clinical recovery: 1d shorter
No diff. in SaO2 or viral load
Study stopped early because "outcome would not change with further participant enrolment".
Sampling of 1o: DKA, AKI, ?rib trauma
Sounds great, right? NNT=8!
Clinical recovery: 1d shorter
No diff. in SaO2 or viral load
Study stopped early because "outcome would not change with further participant enrolment".
Sampling of 1o: DKA, AKI, ?rib trauma
Why was study halted? Reduced recruitment after national lockdown, vaccination, and "ethical consideration of the primary outcome".
Funding: NIHR and AstraZeneca
Make your own conclusion, but unblinded, small study, with no substantial and convincing evidence of real pt. benefit
Funding: NIHR and AstraZeneca
Make your own conclusion, but unblinded, small study, with no substantial and convincing evidence of real pt. benefit
So let& #39;s look at PRINCIPLE, an open-label, adaptive platform
Adults in UK, recruited in Primary Care
≥65 or ≥50 w/comorbidities
New-onset (<14d) COVID symptoms
Not all PCR+
Budesonide inh 800mcg bid x 14d vs. azithro, doxy (1st 17d), colchicine (last 27d), or usual care.
Adults in UK, recruited in Primary Care
≥65 or ≥50 w/comorbidities
New-onset (<14d) COVID symptoms
Not all PCR+
Budesonide inh 800mcg bid x 14d vs. azithro, doxy (1st 17d), colchicine (last 27d), or usual care.
1o outcome: initially hosp/death --> time to pt. self-reported "feeling recovered" and hosp/death d/t COVID.
Recovery used Bayesian exponential model (regressed on Rx and stratification), including parameters for temporal drift. @DrToddLee
Hosp/death didn& #39;t incl. temporal drift.
Recovery used Bayesian exponential model (regressed on Rx and stratification), including parameters for temporal drift. @DrToddLee
Hosp/death didn& #39;t incl. temporal drift.
Trial was stopped (as with STOIC) because UK had done such a good job with public health measures and vaccination, so unlikely to get closer to answering hospitalization/death question.
Analysis is an interim analysis up to March 25.
N=1032 budesonide, 1943 usual care
Analysis is an interim analysis up to March 25.
N=1032 budesonide, 1943 usual care
Analysis provided incl. only 751 (budesonide), 1028 (usual), and 643 (other) PCR+. (i.e. not an ITT)
Mean age: 63, 83% with comorbidities.
Time to first recovery HT 1.2 (~3 days).
8.5% (59/692) vs 10.3% (100/968) hospitlzn/death, but an additional 2 hospitalzn in budesonide group
Mean age: 63, 83% with comorbidities.
Time to first recovery HT 1.2 (~3 days).
8.5% (59/692) vs 10.3% (100/968) hospitlzn/death, but an additional 2 hospitalzn in budesonide group
Oy! What to do? It all sounded so clean at first!
But doesn& #39;t it make sense to give budesonide to all?
1. We have done this "what harm could it cause" before with hydroxychloroquine, ivermectin, etc. (Guess what, it caused problems)
2. There is reason to believe ...
But doesn& #39;t it make sense to give budesonide to all?
1. We have done this "what harm could it cause" before with hydroxychloroquine, ivermectin, etc. (Guess what, it caused problems)
2. There is reason to believe ...
that inhaled steroids increase risk of bacterial pneumonia. ( https://www.bmj.com/content/363/bmj.k4388).">https://www.bmj.com/content/3... Relevant with short duration? Dunno.
Also, at least in UK, inh steroids was actually associated w/worse outcomes not better: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30415-X/fulltext#.YIrLr7Qf5MU.twitter">https://www.thelancet.com/journals/...
Also, at least in UK, inh steroids was actually associated w/worse outcomes not better: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30415-X/fulltext#.YIrLr7Qf5MU.twitter">https://www.thelancet.com/journals/...

 Read on Twitter
Read on Twitter