Anvisa (Brazil& #39;s drug regulator) has just announced it has denied the request to import Sputnik V vaccine because of absence of data & issues with the development of the vaccine, including vax quality. https://g1.globo.com/bemestar/vacina/ao-vivo/reuniao-anvisa-vacina.ghtml">https://g1.globo.com/bemestar/... Meeting still streaming https://www.youtube.com/watch?v=loqGRfjIq8c">https://www.youtube.com/watch... ..1/n
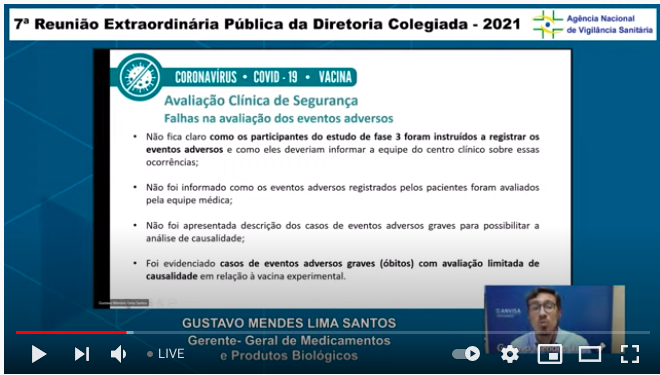
...Unanswered questions about the vaccine include basic biological data about its actions in the body, data on adverse events (including questions about thromboembolic events) ...2/n
...I think with all the focus on Lancet paper for phase 3, people forget how little study there had been of this vax: eg only 38 people in phase 1/2 trial version with lots of problems. ( https://absolutelymaybe.plos.org/2020/09/11/phase-3-results-in-sight-for-some-while-one-vaccine-is-put-on-hold-covid-19-vaccine-race-month-9/)">https://absolutelymaybe.plos.org/2020/09/1... Assessing a vaccine isn& #39;t only about the phase 3 trial results...3/n
...They pointed to issues with reporting of adverse events in the phase 3 trial (I wrote about this here: #Sputnik">https://absolutelymaybe.plos.org/2021/03/16/community-impact-data-3-new-covid-vaccines-and-trials-in-children-a-month-of-dilemmas-and-good-news/ #Sputnik).">https://absolutelymaybe.plos.org/2021/03/1... Remember: deficiencies in a trial/study program are not the same as deficiencies in a vax, but they affect how certain we can be about the vax...4/n
..*So* wishing I understood Portuguese properly right now! Big thanx to @gutoaqui who& #39;s translating these slides as I post them.
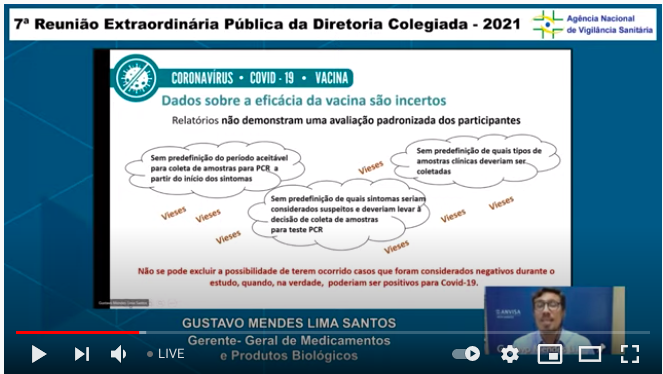
Here pointing to uncertainties around vax efficacy because of biases that could arise from the way they ascertained & determined cases of Covid-19..5/n
Here pointing to uncertainties around vax efficacy because of biases that could arise from the way they ascertained & determined cases of Covid-19..5/n
... This is depressing. Basically, the developers have failed to provide enough data to back up their claims of efficacy & have left a string of open questions about te vaccine& #39;s development. ...6/n
...Questions about quality control of manufacturing. (I& #39;m now at the point I& #39;m glad I don& #39;t understand enough Portuguese - this is too depressing) ...7/n
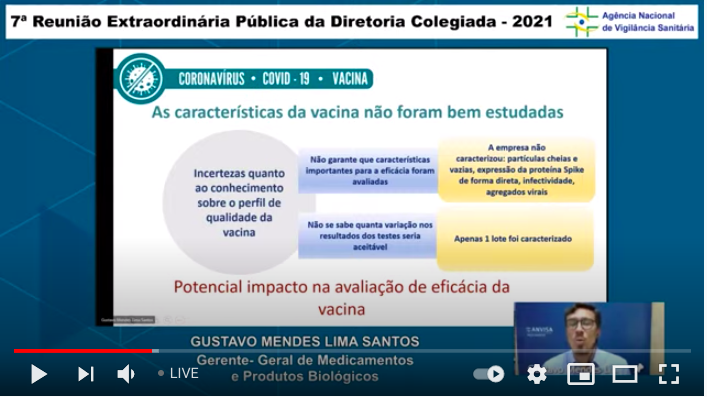
...Distilling some of the fundamental issues lacking here: assurance of potency of the formulation (if I understand that correctly) & basic issues about how replication for the adenoviral vectors in both shots...8/n
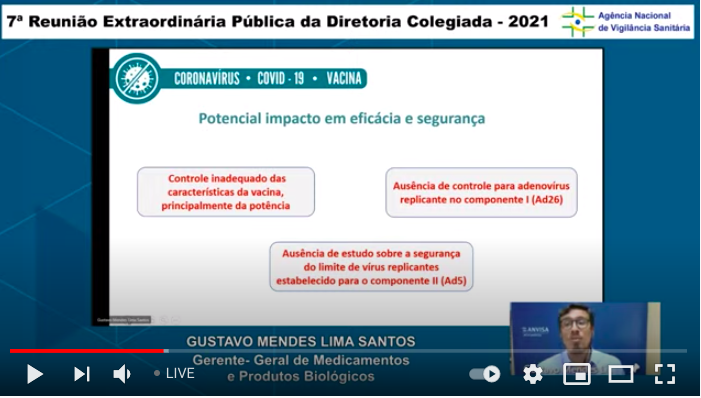
...The catalog of what& #39;s missing to explain the action of these vaccines continues: here basics about spike protein & replication of the Ad26 component (the 1st dose of Sputnik V). (I& #39;m only including some of the slides, not all of them) ...9/n
...Onto trying to establish the credentials of the manufacturing sites: couldn& #39;t, if I& #39;m understanding this correctly.
I& #39;ve seen enough. This seems very thorough work again from Anvisa. For those who want to see more, a reminder it& #39;s here https://www.youtube.com/watch?v=loqGRfjIq8c">https://www.youtube.com/watch... 10/10
I& #39;ve seen enough. This seems very thorough work again from Anvisa. For those who want to see more, a reminder it& #39;s here https://www.youtube.com/watch?v=loqGRfjIq8c">https://www.youtube.com/watch... 10/10
@gutoaqui Thank you so much!
PS: The adenovirus in Covid vax is not supposed to replicate (it& #39;s supposed to be just a carrier). Specific critical detail on Anvisa& #39;s Sputnik V evaluation I missed: replicating adenovirus was identified in every lot of the vax inspected https://coronavirus.atarde.com.br/diretoria-da-anvisa-rejeita-importacao-e-uso-da-sputnik-v/">https://coronavirus.atarde.com.br/diretoria... HT @swiftshoes
Important PPS: the Ad26 vax lots (first Sputnik V shot) not yet analyzed - this is what was identified in every lot of the Ad5 vax (2nd shot) HT @Dereklowe https://twitter.com/DavidRach2/status/1387183627193229313">https://twitter.com/DavidRach...

 Read on Twitter
Read on Twitter