An interesting contrast of the @US_FDA label for tafamidis (in ATTR-CM) vs daratumumab (in AL).
NYHA class IV were excluded in ATTRACT and NYHA class IIIb and IV excluded in ANDROMEDA.
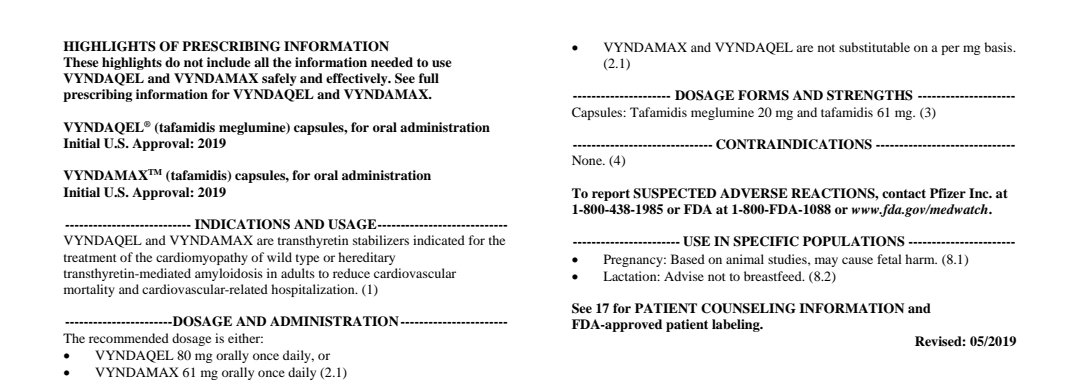
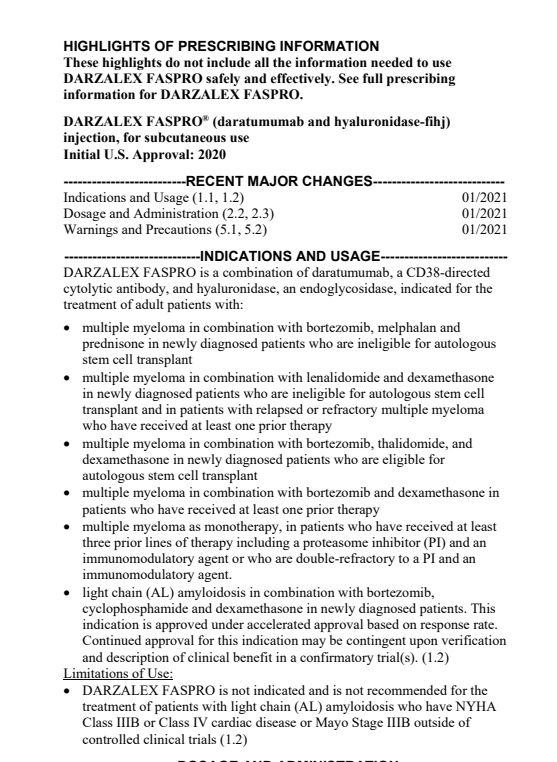
Label for tafamidis without restrictions, daratumumab with
#cardiotwitter #amyloidosis
NYHA class IV were excluded in ATTRACT and NYHA class IIIb and IV excluded in ANDROMEDA.
Label for tafamidis without restrictions, daratumumab with
#cardiotwitter #amyloidosis
This is likely driven by the safety profile in ANDROMEDA and with the FDA having access to the data
---------
Label for tafamidis
https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.fda.gov/media/126283/download&ved=2ahUKEwjtoIPNiJjwAhXBFjQIHQ1KBaQQFjAAegQIBBAC&usg=AOvVaw3O_BSgebx6MoS6vbsDWC43">https://www.google.com/url...
---------
Label for tafamidis
https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.fda.gov/media/126283/download&ved=2ahUKEwjtoIPNiJjwAhXBFjQIHQ1KBaQQFjAAegQIBBAC&usg=AOvVaw3O_BSgebx6MoS6vbsDWC43">https://www.google.com/url...
Label for daratumumab- last section "limitation of use"
https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761145s002lbl.pdf&ved=2ahUKEwiUw8rmipjwAhXcCTQIHTsqD_oQFjADegQIFRAC&usg=AOvVaw3ePu9NH4D0u9YUMab7BtMD">https://www.google.com/url...
https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761145s002lbl.pdf&ved=2ahUKEwiUw8rmipjwAhXcCTQIHTsqD_oQFjADegQIFRAC&usg=AOvVaw3ePu9NH4D0u9YUMab7BtMD">https://www.google.com/url...
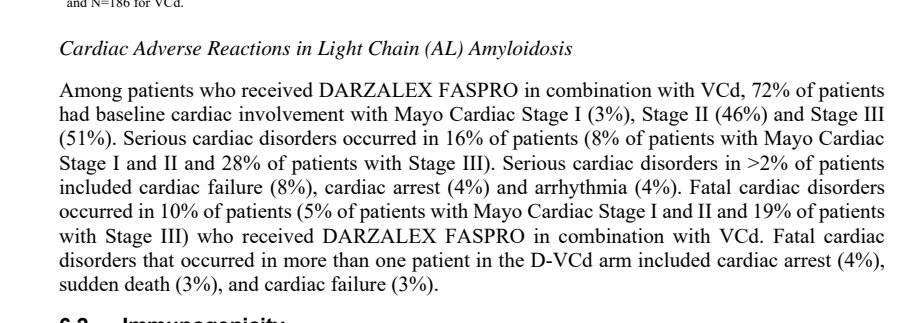
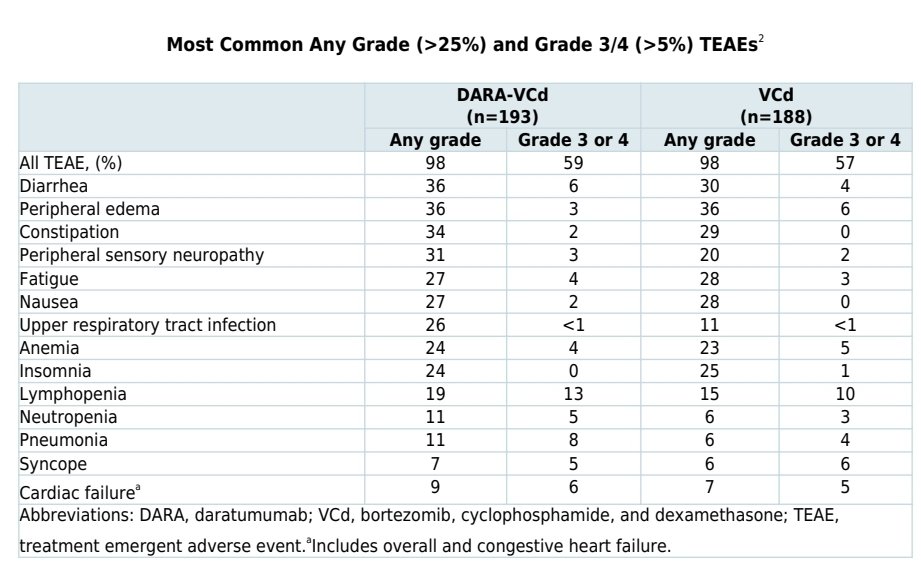
If you read further in the fine print, in ANDROMEDA there was a slight imbalance in the risk of arrhythmias. The FDA label does not dive into the specifics of why they are emphasizing the adverse cardiac events in the dara arm instead of comparing it to the VCd arm.
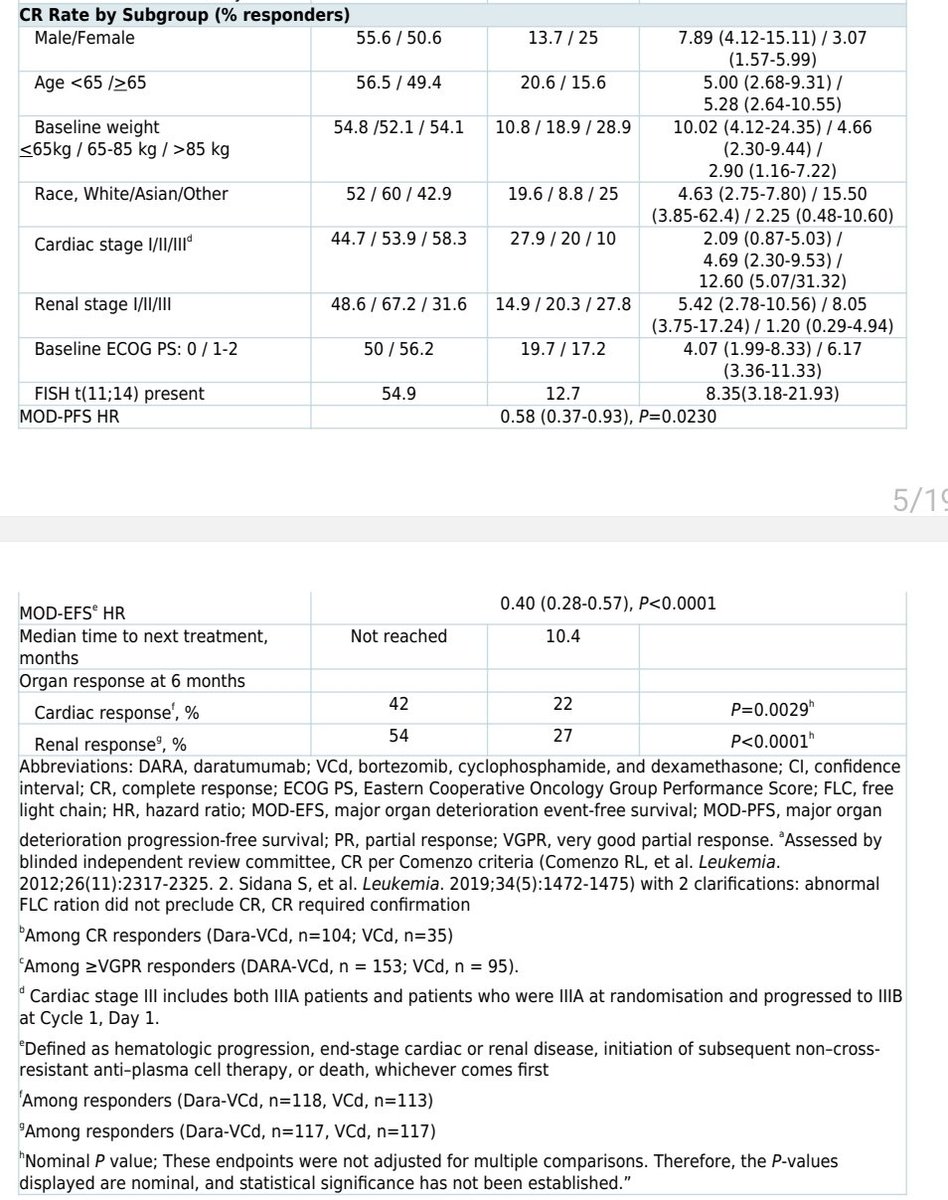
In the ANDROMEDA data, a slight imbalance in heart failure but also a much higher cardiac response.
https://www.janssenmd.com/printpdf/darzalex-faspro/clinical-data/amyloidosis/amy3001-andromeda-study?pdf-version=">https://www.janssenmd.com/printpdf/...
https://www.janssenmd.com/printpdf/darzalex-faspro/clinical-data/amyloidosis/amy3001-andromeda-study?pdf-version=">https://www.janssenmd.com/printpdf/...
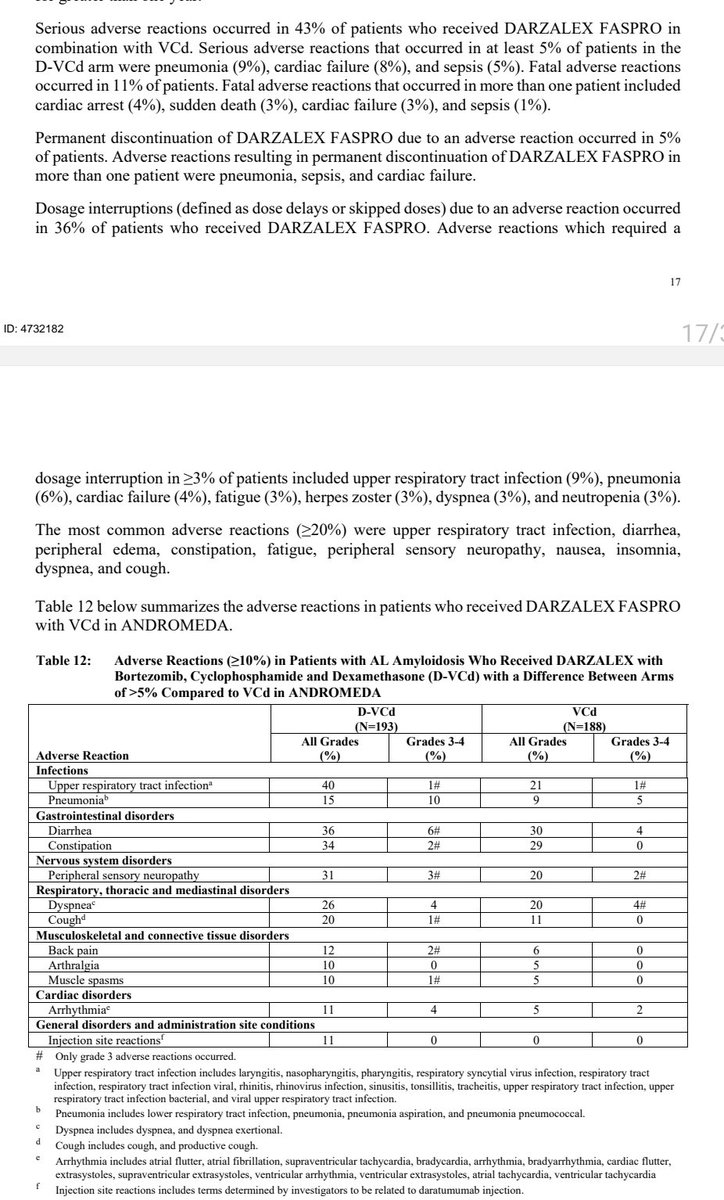
Numbers are small, and there is not a strong signal for adverse cardiac outcomes in AL patients treated with daratumumab+CyBorD. What& #39;s interesting is that they way the label is written, it gives insurance companies an easy out in some markets to deny daratumumab for select pts.
I have seen a recent denial for daratumumab based on an NTproBNP of ~8550 despite NYHA class IIIa. In such a rare disease, we would allow certain entities to deny treatment based on crude measures. An NTproBNP of 7800 is not that different from 8500.
And the right diuretic regimen + treating the light chains can move patients to earlier NYHA classes.

 Read on Twitter
Read on Twitter