1. I& #39;m covering ACIP& #39;s meeting on J&J& #39;s vaccine. Won& #39;t have time to tweet a lot. @CDCgov vaccine safety expert Tom Shimabukuro is up now. Going to present new data on the number of events in the US. Up to 15 events, 3 deaths. Streaming here: https://www.ustream.tv/channel/VWBXKBR8af4">https://www.ustream.tv/channel/V...
2. Shimabukuro says so far no reports of TTS — Thrombosis with Thrombocytopenia Syndrome — have been reported in relation to use of the mRNA vaccines.
3. So far all the cases of TTS linked to the J&J vaccine have been in women. But Shimabukuro said that there are additional possible cases being evaluated, some among males. Cannot assume there are no cases in men. (Not clear why the male case from the J&J trial isn& #39;t included.)
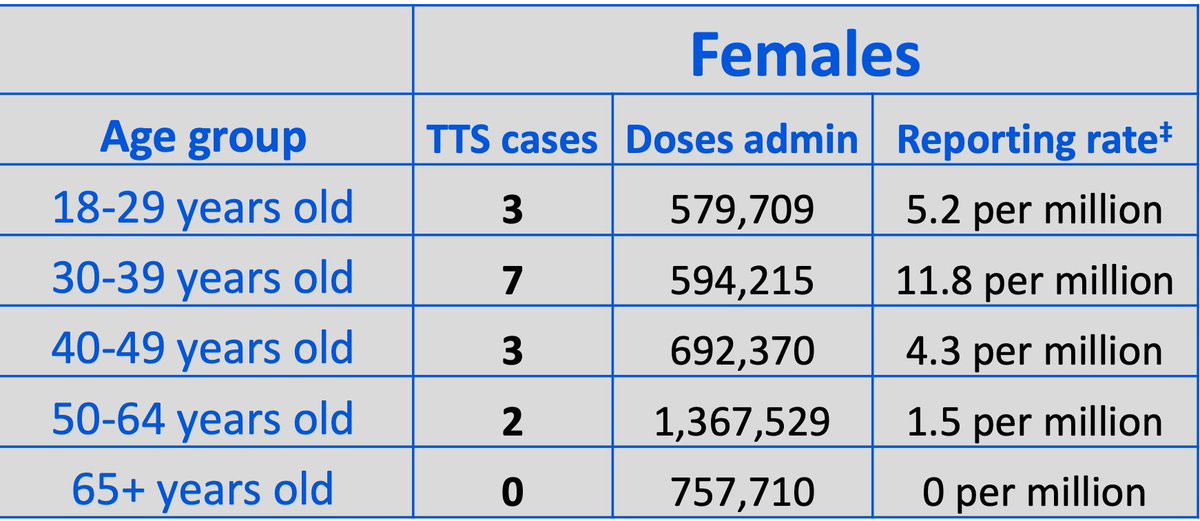
4. About 4M of the J&J doses used so far were given to women. The risk seemed highest among women in their 30s. (It& #39;s early days. A few additional cases in another age group could change this picture quickly.)
5. Shimabukuro was asked how many possible TTS cases are being evaluated. Wouldn& #39;t give a specific number; says it changes daily. But said it& #39;s "just under 10" right now.
6. Mathia Mammen, J&J& #39;s global head for R&D for vaccines is now presenting. J&J isn& #39;t challenging the existence of TTS. Supports including information on it in the vaccine labeling information.
7. Forgot to mention something Tom Shimabukuro revealed earlier. None of the 3 people who died with TTS after receipt of the J&J vaccine were treated with heparin.
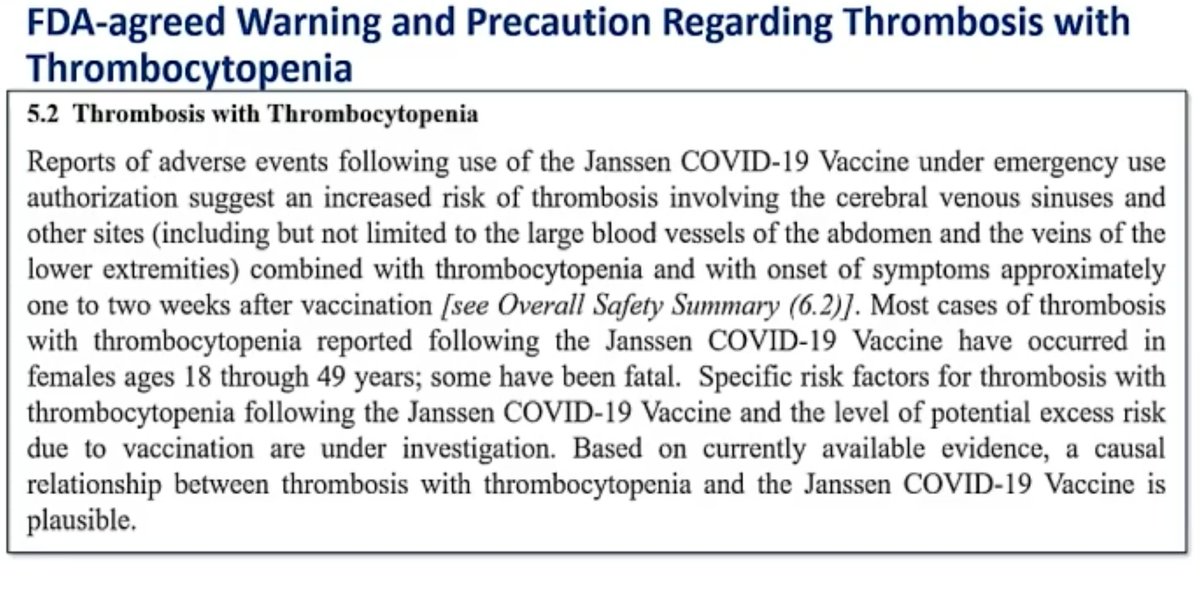
8. This is the warning label language that J&J has agreed to with FDA.
Unless ACIP votes against lifting the pause, this means this vaccine will be coming back into use.
Unless ACIP votes against lifting the pause, this means this vaccine will be coming back into use.
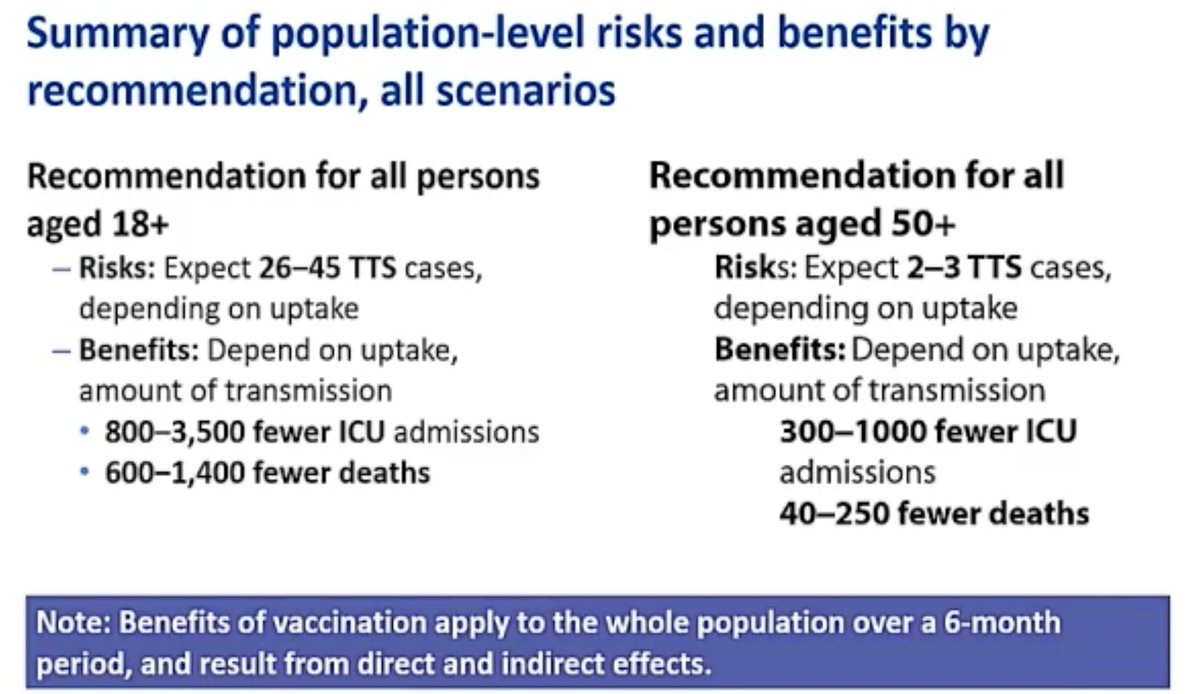
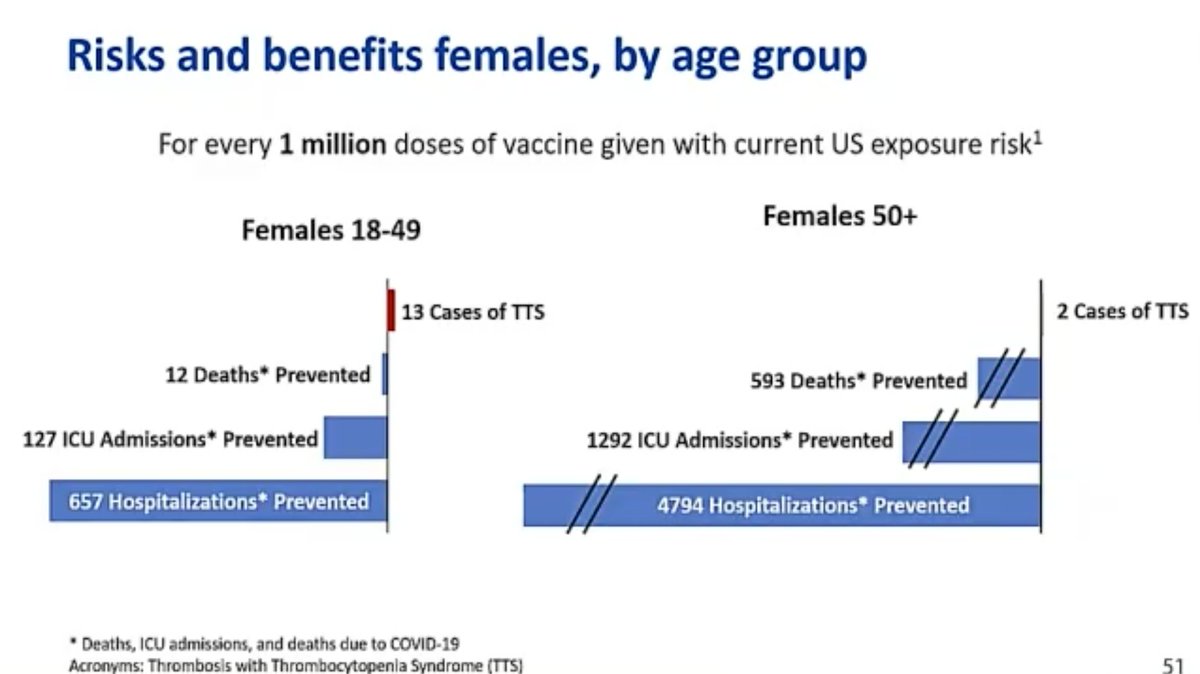
9. J&J& #39;s chief medical officer, Joanne Waldstreicher presented a risk-benefits evaluation. For every 1M people vaxed with the J&J vaccine, 2000 fewer people would die from Covid & 6000 fewer would be hospitalized. There would be 2 cases of TTS expected in this scenario.
10. Waldstreicher said if 1M women under 50 were vaccinated with the J&J vaccine, there would be 116 fewer Covid deaths and 2638 fewer Covid hospitalizations. Under this scenario, 7 cases of TTS would be expected.
11. One of the central characteristics of ACIP meetings is that they are public. The committee doesn& #39;t just crunch data & debate decisions, it shows its work. Some of the presentations are about showing how the investigation has been conducted.
12. Interesting factoid: Women aged 18-29 make up the largest group of people who& #39;ve been infected with Covid since the beginning of March. From a presentation by @cdcgov& #39;s Sara Oliver.
13. Oliver tells ACIP members: You& #39;ll need to think about the population level risk-benefit analysis & the individual level risk-benefit analysis
Therein lies the rub.
Therein lies the rub.
14. What& #39;s that look like? This.
16. (Broke my thread. Repeating to fix.)
What is ACIP going to recommend today? Here are the options presented to them, from ACIP& #39;s Covid vaccine work group. Almost certainly going to be one of these.
No debate yet, so can& #39;t say how the committee is leaning. But watch #4.
What is ACIP going to recommend today? Here are the options presented to them, from ACIP& #39;s Covid vaccine work group. Almost certainly going to be one of these.
No debate yet, so can& #39;t say how the committee is leaning. But watch #4.
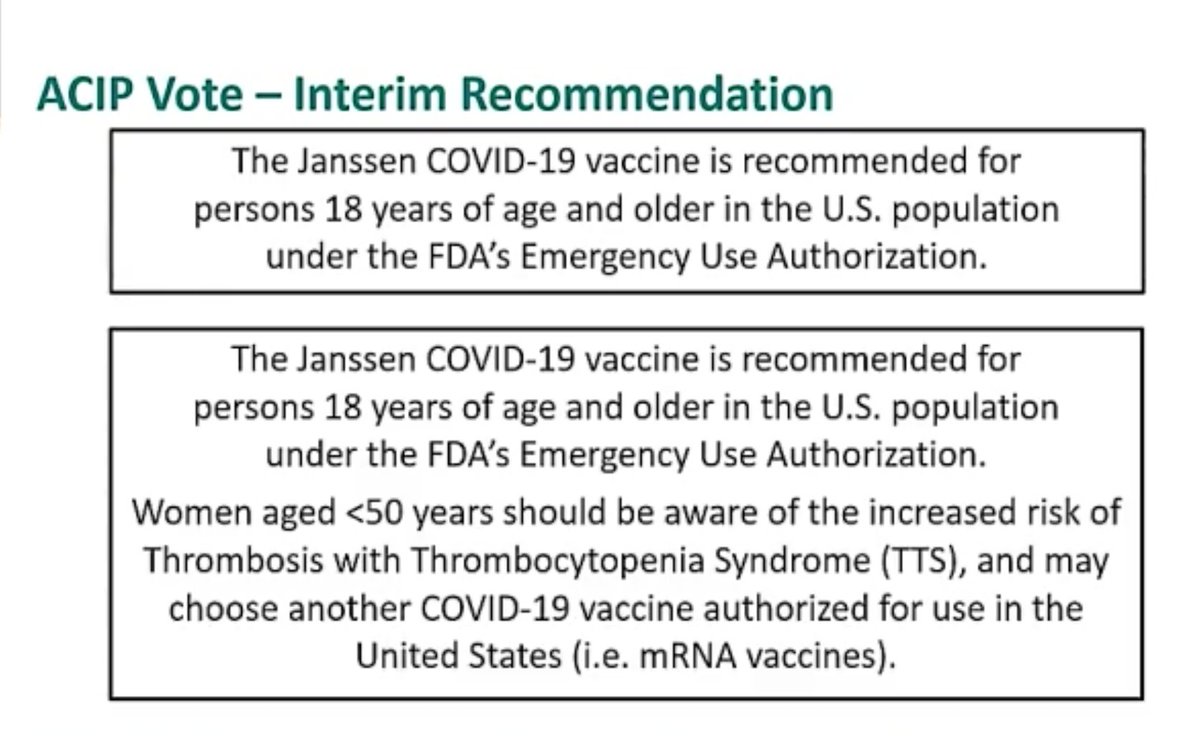
17. It& #39;s still early in the discussion period but so far support seems to be coalescing around option #4.
18. @DocJeffD raises an important point: The risk-benefit analyses are based on current levels of risk. But as more people get vaccinated, the risk will drop, changing the equation. @CDCgov staff said they will continue to update the model.
19. Beth Bell, chair of ACIP& #39;s Covid vaccine work group, stressed that the decision taken today is to come up with a policy for now, not for always. (This gets to @DocJeffD& #39;s comment about the risk-benefit ratio changing when Covid transmission is lower in the US.)
20. Listening to ACIP& #39;s deep dive into risks & benefits of the J&J vaccine reminds one of how amazing the US advisory committee system for HHS agencies is. VRBPAC (FDA) & ACIP (CDC) kick tires in public. This is unique and very valuable.
21. Late afternoon shift — sounds like the committee may be veering towards option #2, not #4. Options 1 & 3 are off the table.
22. Having some internal (STAT) discussions about what& #39;s going on in ACIP right now. @matthewherper described it as "they are debating how to warn without warning."
Feels right. And kind of confusing.
Feels right. And kind of confusing.
25. The motion passed 10 for, 4 against, 1 abstention (because the member is involved in clinical trials).

 Read on Twitter
Read on Twitter