This week marks  https://abs.twimg.com/emoji/v2/... draggable="false" alt="1⃣" title="Tastenkappe Ziffer 1" aria-label="Emoji: Tastenkappe Ziffer 1">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="1⃣" title="Tastenkappe Ziffer 1" aria-label="Emoji: Tastenkappe Ziffer 1"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="5⃣" title="Tastenkappe Ziffer 5" aria-label="Emoji: Tastenkappe Ziffer 5"> years of biosimilar medicines in Europe. They have greately contributed to better access across Europe and beyond. Thread
https://abs.twimg.com/emoji/v2/... draggable="false" alt="5⃣" title="Tastenkappe Ziffer 5" aria-label="Emoji: Tastenkappe Ziffer 5"> years of biosimilar medicines in Europe. They have greately contributed to better access across Europe and beyond. Thread  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> #biosimilars #access4all https://twitter.com/medicinesforEU/status/1381555506960027651">https://twitter.com/medicines...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> #biosimilars #access4all https://twitter.com/medicinesforEU/status/1381555506960027651">https://twitter.com/medicines...
In  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇪" title="Flagge von Deutschland" aria-label="Emoji: Flagge von Deutschland">Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇪" title="Flagge von Deutschland" aria-label="Emoji: Flagge von Deutschland">Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU
A milestone has been the introduction of #biosimilar adalimumab. In  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇰" title="Flagge von Dänemark" aria-label="Emoji: Flagge von Dänemark">Denmark, with introduction of ada biosimilars, they have managed a national switch in just 3 weeks for 90% of use! Saved DKK 350 million in 2019 alone. https://bit.ly/3daEuFw ">https://bit.ly/3daEuFw&q...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇰" title="Flagge von Dänemark" aria-label="Emoji: Flagge von Dänemark">Denmark, with introduction of ada biosimilars, they have managed a national switch in just 3 weeks for 90% of use! Saved DKK 350 million in 2019 alone. https://bit.ly/3daEuFw ">https://bit.ly/3daEuFw&q...
Several biologics that have biosimilar versions are being added to @WHO essential medicines list and prequalified. These are esential treatments such as insulins https://bit.ly/3wRd5jS ">https://bit.ly/3wRd5jS&q... Very important for greater access globally.
There is much more to be done - lack of uptake in CEE region - also where we can observe issues with access to biologic treatments. Comprehensive uptake policies could make a big difference. Check out @medicinesforEU marker review 2020 https://bit.ly/3uJFA10 ">https://bit.ly/3uJFA10&q...
If you are interested to learn more about early days read @Julie_MarJam blog with perspectives of different stakeholders, The beginning of biosimilar medicines journey https://bit.ly/3mHYEK9 ">https://bit.ly/3mHYEK9&q...
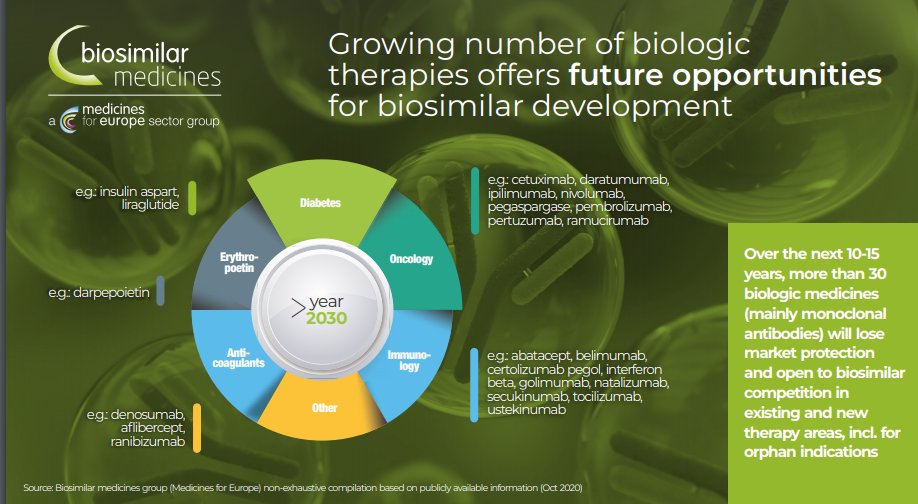
And finally, future perspective: By 2025, over 70% of new meds registered will be biologics. This is why it will be important to optimise the current framework and support introduction to markets as indicated in plans by @EMA_News and @EU_Health to achieve #access4all

 Read on Twitter
Read on Twitter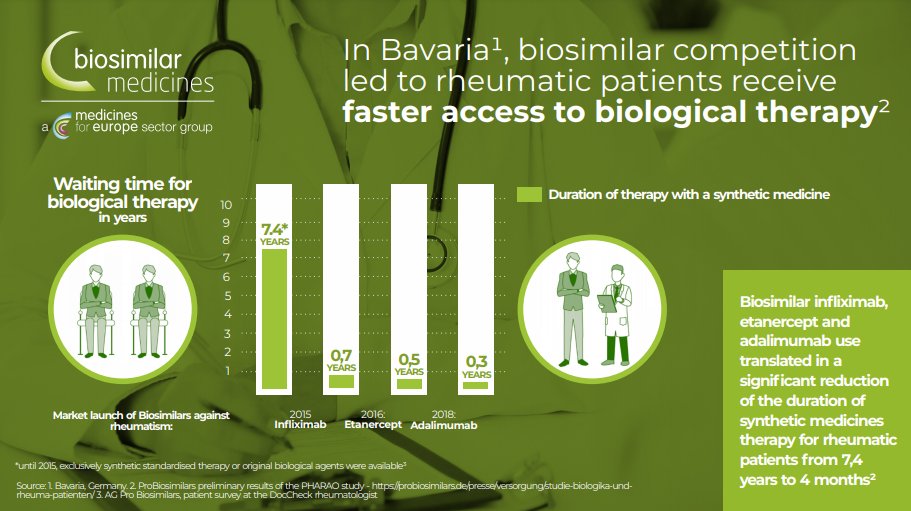 Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU" title="In https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇪" title="Flagge von Deutschland" aria-label="Emoji: Flagge von Deutschland">Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU" class="img-responsive" style="max-width:100%;"/>
Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU" title="In https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇩🇪" title="Flagge von Deutschland" aria-label="Emoji: Flagge von Deutschland">Germany , today patients with rheumatoid arthritis get access to biologics in 0.3 years in comparison to 7.4 years before #biosimilars were introduced. @ProBiosimilars @medicinesforEU" class="img-responsive" style="max-width:100%;"/>