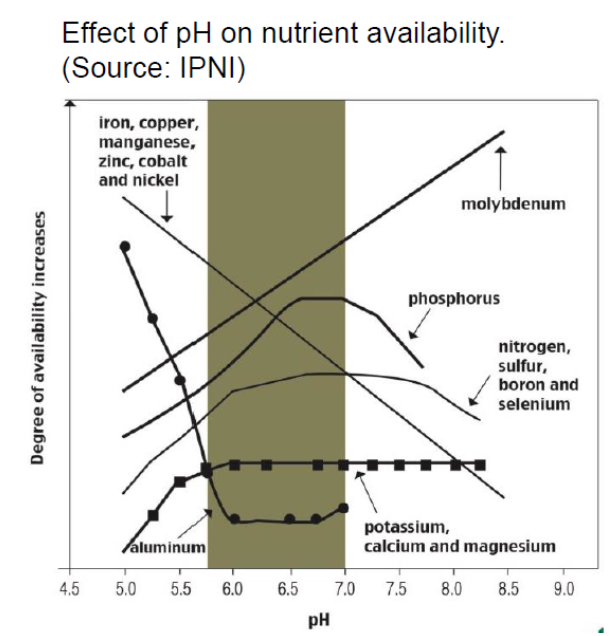
I was curious to see where mindset was with this, and not surprised by the very mixed results. We often have a bias towards thinking "deficiencies" with nutrients, but we need to think equally about toxicities as well, and Mn is one that can often be toxic at low pH. https://twitter.com/fielddirt/status/1381013040011747328">https://twitter.com/fielddirt...
Here& #39;s a little more info on Mn. Note that in high pH soil, especially if sandy and in oats, Mn def is definately a possibility. Also organic soils are potentially a problem even with lower pH. #39;s%20needles.-,Toxicity,soil%20pH%20is%20below%206.0.&text=may%20accumulate%20very%20high%20levels,the%20required%20low%20soil%20pH">https://spectrumanalytic.com/support/library/ff/Mn_Basics.htm#:~:text=current%20season& #39;s%20needles.-,Toxicity,soil%20pH%20is%20below%206.0.&text=may%20accumulate%20very%20high%20levels,the%20required%20low%20soil%20pH.">https://spectrumanalytic.com/support/l...
But the reality we see in very acid mineral soils is that the potential for toxic levels of Mn is far, far higher than deficiencies. And wet soils can make this worse as we saw last year in northern AB. See original thread for some great pics from partners.
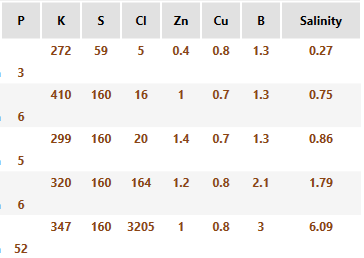
I could argue that chloride toxicities are also quite common in poorly drained, salt affected areas - I& #39;ve seen 1000& #39;s of lbs of Cl in SWAT zone 10s. But it& #39;s salts in general, not just Cl. Do you really want to apply any potash in those areas??
Boron is another nutrient that we could be seeing at toxic soil levels, again in poorly drained, usually higher organic matter depression areas, testing in excess of 5 ppm occasionally. Interestingly the same field can have well drained, potentially deficient areas (0.5 ppm)
Boron toxicity is well documented in poorly drained sodic soils in AUS, and I suspect we can find the same thing here in western Canada. http://soilquality.org.au/factsheets/boron">https://soilquality.org.au/factsheet...
The point is, superfluous use of nutrients can occasionally do more harm than good, and isn& #39;t necessarily easy to recognize.
Understand your soil, and beware of marketing gimmicks with fertilizers!
Understand your soil, and beware of marketing gimmicks with fertilizers!

 Read on Twitter
Read on Twitter