The most important vaccine in Latin America—SinoVac’s CoronaVac reports new trial results from Brazil  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—
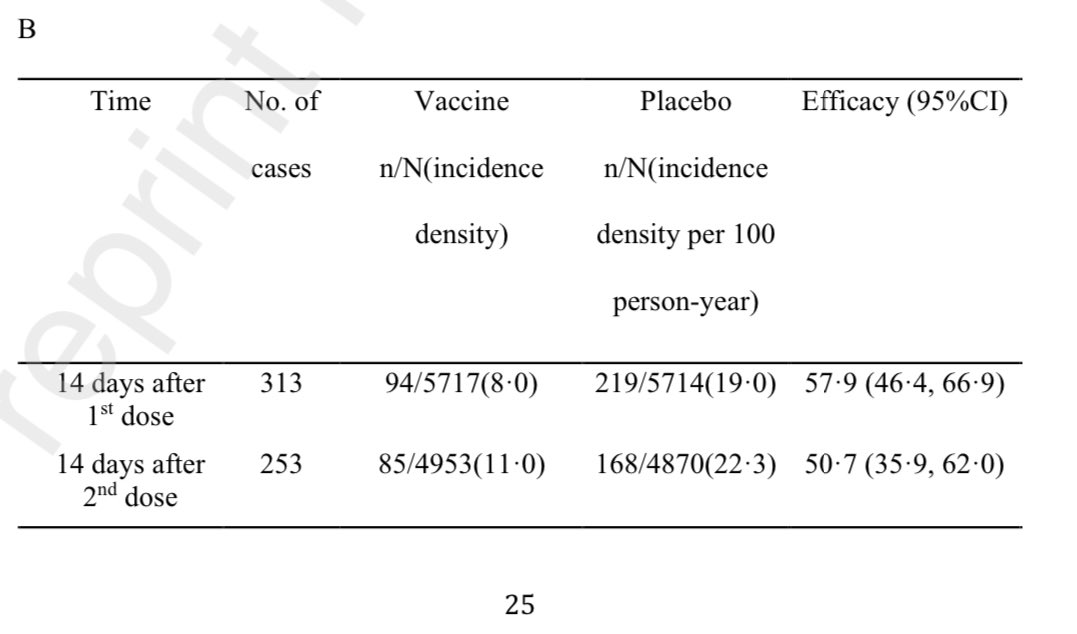
Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1. https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3822780">https://papers.ssrn.com/sol3/pape...
Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1.
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3822780">https://papers.ssrn.com/sol3/pape...
2) Results 12,396 people randomized in Brazil. It seems 2nd dose efficacy wasn’t much diff than 14 days after 1st dose. And delaying 2nd dose didn’t seem to hurt. And a second dose >21 days actually showed no drop in efficacy.
3) As for lab antibody neutralization titres, vaccinated people had no real difference for seroconverting (having antibodies) against different variants. That said, among those who seroconverted, neutralization titres was lower among those age 60 and above against #P1  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant.
4) Overall though, there was no real efficacy difference by age. But keep in mind — this trial was mostly conducted months ago before the large nationwide surge of the #P1 across Brazil  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge.
8) But there was oddly a significantly 13% relative higher relative rate of cardiovascular disease in the vaccine group (70.5% vs 62.3%) among those with comorbidities. What’s up with that? There was no discussion in the paper. It seems to maybe mix prevalence + new incidence?
9) I don’t know what to make of the slightly higher obesity in vaccines. Again, the % is high, so maybe it mixed both existing prevalence plus new onset? This needs an explanation.
10) And to be clear—the authors say #P1 was not observed in the study (like I said before, trial was mostly before the nationwide surge). Only #P2 (the other Brazil  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant) was observed.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant) was observed.
11) But good news is that seroconversion was equal between the variants. It’s could be because CoronaVac is an inactivated virus—ie full presentation of (dead) virus, not just the spike protein. Some experts theorize this could make CoronaVac better against variants. Unclear.
12) Yes, the 50% efficacy is much lower than the 95% efficacy seen with mRNA vaccines. But maybe the redeeming quality maybe that inactivated vaccine could be better against variants! We’ll see. https://apnews.com/article/beijing-immunizations-chengdu-coronavirus-pandemic-china-675bcb6b5710c7329823148ffbff6ef9">https://apnews.com/article/b...
13) To be fair, the trial results do confirm another observational study (not trial) in Manaus when it had 75% #P1. It also found 50% efficacy. However it dropped to 35% when asymptomatic cases added. See  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> below. https://twitter.com/drericding/status/1380015935906590721">https://twitter.com/drericdin...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> below. https://twitter.com/drericding/status/1380015935906590721">https://twitter.com/drericdin...
14) Previously, SinoVac reported 83.5% efficacy in Turkish trial. And 65% efficacy in Indonesia rural. https://www.reuters.com/article/health-coronavirus-turkey-sinovac-int-idUSKBN2AV18P">https://www.reuters.com/article/h...
15) I do want to point out that efficacy could differ a lot across vaccines due to other reasons like local spread and by type of vaccines. For mRNA ones— Pfizer & Moderna, they reported difference neutralization (not efficacy) by variants. #P1 was moderately poor. #B1351 worst. https://twitter.com/drericding/status/1371209487491735553">https://twitter.com/drericdin...
16) CoronaVac has also previously reported favorable immune results in kids 3-17. “antibody levels triggered by Sinovac’s CoronaVac were higher than those seen in adults aged 18 to 59 and in elderly people in earlier clinical trials” https://www.google.com/amp/s/mobile.reuters.com/article/amp/idUSKBN2BE2M7">https://www.google.com/amp/s/mob...
17) Wanna know what is better than merely Phase 1/2 results? Real efficacy results of ~100% in teenagers 12-15 for Pfizer!
Even better? Hopefully FDA approval soon! https://twitter.com/drericding/status/1380576503440752645">https://twitter.com/drericdin...
Even better? Hopefully FDA approval soon! https://twitter.com/drericding/status/1380576503440752645">https://twitter.com/drericdin...
18) CoronaVac is so all over the place. Here is a Chilean study.
“protection with only 1 dose is estimated to be 3%; for those with 2 doses with less than 14 days is 27.7%; and 56.5% effectiveness for those who have the second dose more than 14 days ago.” https://www.emol.com/noticias/Nacional/2021/04/06/1017076/desafios-primer-estudio-vacunacion-chile.html">https://www.emol.com/noticias/...
“protection with only 1 dose is estimated to be 3%; for those with 2 doses with less than 14 days is 27.7%; and 56.5% effectiveness for those who have the second dose more than 14 days ago.” https://www.emol.com/noticias/Nacional/2021/04/06/1017076/desafios-primer-estudio-vacunacion-chile.html">https://www.emol.com/noticias/...

 Read on Twitter
Read on Twitter —Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://papers.ssrn.com/sol3/pape..." title="The most important vaccine in Latin America—SinoVac’s CoronaVac reports new trial results from Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://papers.ssrn.com/sol3/pape..." class="img-responsive" style="max-width:100%;"/>
—Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://papers.ssrn.com/sol3/pape..." title="The most important vaccine in Latin America—SinoVac’s CoronaVac reports new trial results from Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—Efficacy against symptomatic #COVID19–50.7%. Moderate & severe: 83.7% / 100%. Delayed 2nd dose no issue. Neut. titres lower in age>=60 against #P1.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://papers.ssrn.com/sol3/pape..." class="img-responsive" style="max-width:100%;"/>

 variant." title="3) As for lab antibody neutralization titres, vaccinated people had no real difference for seroconverting (having antibodies) against different variants. That said, among those who seroconverted, neutralization titres was lower among those age 60 and above against #P1 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant." class="img-responsive" style="max-width:100%;"/>
variant." title="3) As for lab antibody neutralization titres, vaccinated people had no real difference for seroconverting (having antibodies) against different variants. That said, among those who seroconverted, neutralization titres was lower among those age 60 and above against #P1 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant." class="img-responsive" style="max-width:100%;"/>
 —this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge." title="4) Overall though, there was no real efficacy difference by age. But keep in mind — this trial was mostly conducted months ago before the large nationwide surge of the #P1 across Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge." class="img-responsive" style="max-width:100%;"/>
—this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge." title="4) Overall though, there was no real efficacy difference by age. But keep in mind — this trial was mostly conducted months ago before the large nationwide surge of the #P1 across Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien">—this trial enrolled people July-Dec 2020, & likely didn’t capture much of #P1 March surge." class="img-responsive" style="max-width:100%;"/>





 variant) was observed." title="10) And to be clear—the authors say #P1 was not observed in the study (like I said before, trial was mostly before the nationwide surge). Only #P2 (the other Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant) was observed." class="img-responsive" style="max-width:100%;"/>
variant) was observed." title="10) And to be clear—the authors say #P1 was not observed in the study (like I said before, trial was mostly before the nationwide surge). Only #P2 (the other Brazil https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇧🇷" title="Flagge von Brasilien" aria-label="Emoji: Flagge von Brasilien"> variant) was observed." class="img-responsive" style="max-width:100%;"/>



