I‘ve had little time this past pandemic year to indulge in my passion for science around the color blue. But I did take some time this week to write about a cool paper describing a potential new blue food dye.
Story is here, quick thread on blue to come: https://www.sciencemag.org/news/2021/04/pigment-red-cabbage-could-help-turn-your-favorite-foods-blue">https://www.sciencemag.org/news/2021...
Story is here, quick thread on blue to come: https://www.sciencemag.org/news/2021/04/pigment-red-cabbage-could-help-turn-your-favorite-foods-blue">https://www.sciencemag.org/news/2021...
Yes, finding new blue food colorants is a whole thing.
The world mostly relies on brilliant blue, E131, and indigotine, E132. Both of these are synthetic, however, and there has been a push by consumers and companies for natural alternatives.
The world mostly relies on brilliant blue, E131, and indigotine, E132. Both of these are synthetic, however, and there has been a push by consumers and companies for natural alternatives.
So the race has been on to find natural alternatives and I‘ve written about this before here:
There is one alternative that is now sometimes used: a crude extract of spirulina algae. #doughnuts-dye">https://www.sciencemag.org/news/2019/05/meet-blue-crew-scientists-trying-give-food-flowers-and-more-color-rarely-found-nature #doughnuts-dye">https://www.sciencemag.org/news/2019...
There is one alternative that is now sometimes used: a crude extract of spirulina algae. #doughnuts-dye">https://www.sciencemag.org/news/2019/05/meet-blue-crew-scientists-trying-give-food-flowers-and-more-color-rarely-found-nature #doughnuts-dye">https://www.sciencemag.org/news/2019...
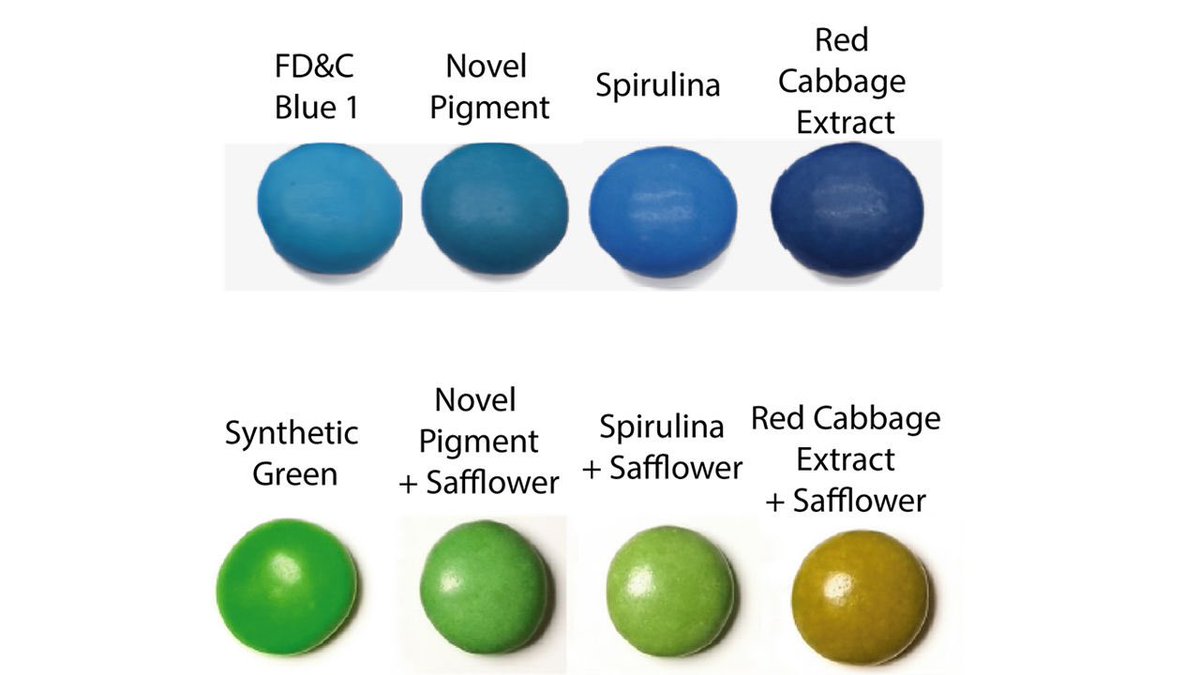
But like many natural blues, spirulina has a lot of purple undertones. That is especially problematic when you want to blend the blue with yellow to get a good green. As @PamDenish tod me: „Purple plus yellow equals brown, so you’re not going to get a very vibrant green.“
Here is an image from the paper comparing different blues and the greens they yield in coatings of sugar lentils:
(The research was done with scientists at Mars Wrigley, so yes this is about the possible future color of your M&Ms too)
(The research was done with scientists at Mars Wrigley, so yes this is about the possible future color of your M&Ms too)
(Ooops. „Real“ work calling. Will continue after.)
Back to blue: In plants the only pigments that can „make“ blue are anthocyanins.
Anthocyanins are fascinating beause they are literally named for „blue flowers“, but mostly in nature we encounter them in red: Take strawberries, red roses or autumn leaves.
Anthocyanins are fascinating beause they are literally named for „blue flowers“, but mostly in nature we encounter them in red: Take strawberries, red roses or autumn leaves.
You can easily turn these molecules blue if you increase the pH, so make their surroundings less acidic: Cut and boil some red cabbage and the water will be purple. Add lemon juice and it turns red. Add baking powder it turns blue.
Most flowers don‘t have this option. So the cornflower does some crazy chemistry to turn its petals blue: It builds huge complexes of anthocyanin molecules plus other molecules arranged around central
metal ions. That stabilizes the „blue“ version of the anthocyanins.
metal ions. That stabilizes the „blue“ version of the anthocyanins.
The researchers of the new paper decided to basically pick one of the anthocyanins of red cabbage that seemed particularly promising, so one part of that blue soup you get from red cabbage and baking powder (there are a lot of slightly different anthocyanins in there).
When they added aluminum ions they found that this particular molecule, they just call it P2, arranged itself in a way reminiscent of the cornflower: In this case three molecules arranged around the central ion. That made the molecule more stable and gave a better blue.
One problem remained: This molecule represents just about 5% of the anthocyanins in red cabbage. Looking for ways to increase yield, the scientists decided to look for enzymes that could convert some of the other anthocyanins in red cabbage into the P2 they wanted.
Indeed they found a bacterial enzyme that did quite well and they designed it to be even better at this through a mutation. After this enzyme has done its job about half the anthocyans of the red cabbage are P2. Pretty cool.
This does not mean, however, that there is going to be new blue M&M‘s soon. For one, the yield is still low: about 75 milligrams of blue from 100 grams of red cabbage.
More importantly, safety and long-term stability of this dye still need to be thoroughly assessed.
More importantly, safety and long-term stability of this dye still need to be thoroughly assessed.
As @bastoslab told me: „the perfect new blue dye must combine: high tinctorial power, broad applicability, low toxicity and environmental impact, low production cost and efficient synthesis, have a hue that people like and perhaps use natural products as starting materials“
So red cabbage may end up giving us a new blue - or it may not.
But it‘s another example of the complexity you can find when you delve into the science and the beauty of blue.
I should know, I wrote a whole book about it, coming out later this year: https://www.amazon.com/Blue-Search-Natures-Rarest-Color-ebook/dp/B08GFF8QHQ">https://www.amazon.com/Blue-Sear...
But it‘s another example of the complexity you can find when you delve into the science and the beauty of blue.
I should know, I wrote a whole book about it, coming out later this year: https://www.amazon.com/Blue-Search-Natures-Rarest-Color-ebook/dp/B08GFF8QHQ">https://www.amazon.com/Blue-Sear...
Paper is out in @ScienceAdvances here: https://advances.sciencemag.org/content/7/15/eabe7871.abstract">https://advances.sciencemag.org/content/7...

 Read on Twitter
Read on Twitter