Today at #VIDS_JC with @justin_denholm ID registrar / fellow Chris Kiss presented “Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia” https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00313-5/fulltext">https://www.thelancet.com/journals/...
Conducted at 16 hospitals in France.
Included adults with moderate community-acquired pneumonia (CAP) who were stable after 72h of β-lactam therapy.
Excluded severe (critical care) CAP, immunosuppressed, suspected atypical organisms, renal failure.
Included adults with moderate community-acquired pneumonia (CAP) who were stable after 72h of β-lactam therapy.
Excluded severe (critical care) CAP, immunosuppressed, suspected atypical organisms, renal failure.
Initial treatment was with β-lactam monotherapy – mainly amox-clav and 3rd generation cephalosporin
Randomisation to either:
oral amox-clav
OR
oral Placebo
for 5 days following the initial 72h of β-lactam therapy
oral amox-clav
OR
oral Placebo
for 5 days following the initial 72h of β-lactam therapy
Primary outcome: cure at day 15 (afebrile, resolution / improvement of signs and symptoms, no further antibiotics)
Non-inferiority margin of 10%. Both ITT and per-protocol population assessed
Non-inferiority margin of 10%. Both ITT and per-protocol population assessed
706 assessed for eligibility, 396 not eligible (122 not clinically stable, 80 severe CAP, 80 renal failure)
310 enrolled
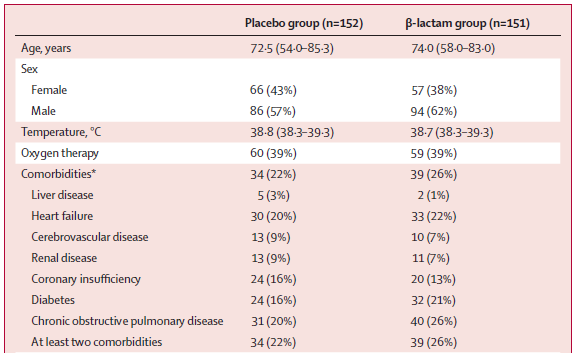
Median pneumonia severity index 82 (PSI class 3)
310 enrolled
Median pneumonia severity index 82 (PSI class 3)
At baseline, table 1 suggests 24% had ≥ 1 comorbidity but I think it& #39;s a typo, and should be 24% had ≥ 2 comorbidities. 21% had heart failure and 23% had chronic obstructive pulmonary disease
Even then, our general experience is patients tend to have multiple comorbidities.
Even then, our general experience is patients tend to have multiple comorbidities.
Therefore, the patients in this trial appear to be less comorbid than other series, borne out for example by looking at:
In the US Jain et al found that 78% of those hospitalised with CAP had an underlying condition. 42% had chronic lung disease, 35% chronic heart disease. The Jain study included patients in ICU so is not directly comparable. https://www.nejm.org/doi/10.1056/NEJMoa1500245">https://www.nejm.org/doi/10.10...
In Australia Lloyd et al found that the median Charlson Comorbidity Index was 3. About 50% had chronic pulmonary disease, and 30% congestive cardiac failure. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2737749">https://jamanetwork.com/journals/...
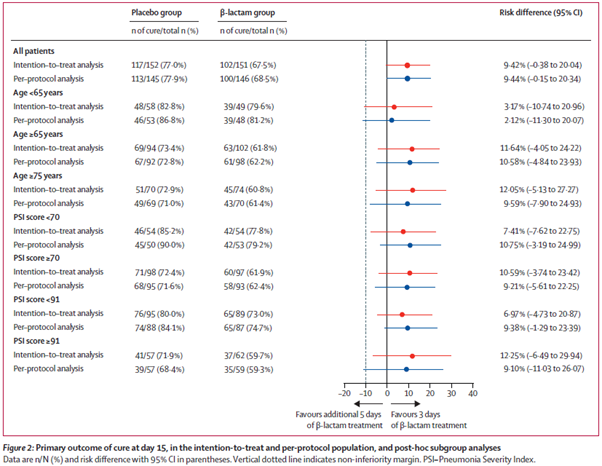
Primary outcome: ITT – 77% cure at day 15 in placebo vs 68% in B-lactam group. Similar in per protocol group. Similar pattern in all pre-specified subgroups.
Strengths – reasonable numbers in the ITT, low numbers lost to follow-up, high rates of adherence, relevant question
The big question is how generally applicable are these findings?
The big question is how generally applicable are these findings?
A. In Australia, recommended initial therapy is IV benzylpenicillin plus doxycycline (or macrolide). In the US fluoroquinolone regimens often first line.
These regimens compared to upfront amox-clav or 3rd gen cephalosporin probably won’t greatly impact upon duration of therapy
These regimens compared to upfront amox-clav or 3rd gen cephalosporin probably won’t greatly impact upon duration of therapy
B. Many patients were not eligible. In the real world, may be even less. It took over 4 years in 16 centers to find 706 patients to be screened and 310 found to be eligible.
Without knowing the exact size of these centers, it does seem to have taken a while for a common disease
Without knowing the exact size of these centers, it does seem to have taken a while for a common disease
Lloyd et al enrolled 816 patients in 2 centers in Australia over 18 months and Jain et al enrolled 2488 patients in 5 hospitals in the US over 2.5 years. So it’s possible that even those screened for eligibility by Dinh et al were already pre-selected.
C. Patients were not as comorbid as expected as noted above. Do these findings apply to a broader group of patients that are more representative of the general CAP population?
D. Diagnostics for an underlying cause were minimal. This is understandable in a pragmatic trial. But it does leave open the possibility that many had a viral infection for which antibiotics are unlikely to be of benefit.
Again, Jain detected a pathogen in 38% of patients, 23% with viruses and 11% with bacteria.
Only 31 of 303 in Dinh et al had positive microbiology. Viral studies were not reported on.
Having a significant number with viral infections would favor a finding of non-inferiority.
Only 31 of 303 in Dinh et al had positive microbiology. Viral studies were not reported on.
Having a significant number with viral infections would favor a finding of non-inferiority.
Take home: The findings are consistent with the broader context of ‘shorter is better’. Most of us in journal club felt that it should be applied to patients similar to those in the trial – fewer comorbidities, clearly improved and stable at day 3.
But this is likely to be a minority of patients with CAP. Further trials could seek to broaden the applicability to a larger set of patients.
Further studies where patients with confirmed viral etiology are excluded are also required to determine if the findings hold true where a bacterial cause is more likely.

 Read on Twitter
Read on Twitter