1/ A liquid biopsy is usually a blood-based test that can detect cancer.
How?
By detecting either CTC (circulating tumor cells) or ctDNA (circulating tumor DNA). In some studies, ctDNA has been shown to have 100x the concentration as CTC though.
How?
By detecting either CTC (circulating tumor cells) or ctDNA (circulating tumor DNA). In some studies, ctDNA has been shown to have 100x the concentration as CTC though.
2/ So why is this important?
Well, the standard of care is tissue biopsies. Doctors lop off a piece of the potentially cancerous area (a chunk of your lung for lung cancer for instance) to verify if cancer exists.
However, tissue biopsies are inferior in a few ways...
Well, the standard of care is tissue biopsies. Doctors lop off a piece of the potentially cancerous area (a chunk of your lung for lung cancer for instance) to verify if cancer exists.
However, tissue biopsies are inferior in a few ways...
3/
1. For one, there are more complications. Lung biopsies had a 19% complication rate.
2. They are also expensive, costing nearly $15,000 for each one.
3. They leave a scar and require a surgery
4. It takes a while to get the results
5. Not a lot of tissue can be retrieved
1. For one, there are more complications. Lung biopsies had a 19% complication rate.
2. They are also expensive, costing nearly $15,000 for each one.
3. They leave a scar and require a surgery
4. It takes a while to get the results
5. Not a lot of tissue can be retrieved
4/ On the other hand, liquid biopsies only require a vial of blood. These genetic tests can profile dozens of genes and determine whether a tumor is malignant.
The main players that already have an approved test are Guardant Health and Foundation Medicine (owned by Roche).
The main players that already have an approved test are Guardant Health and Foundation Medicine (owned by Roche).
5/ However, many companies are jumping into this space. Because Guardant& #39;s LUNAR assay may detect colorectal cancer, Exact Sciences bought Thrive recently for nearly $2.2 billion.
Invitae also bought ArcherDx recently to be competitive.
Invitae also bought ArcherDx recently to be competitive.
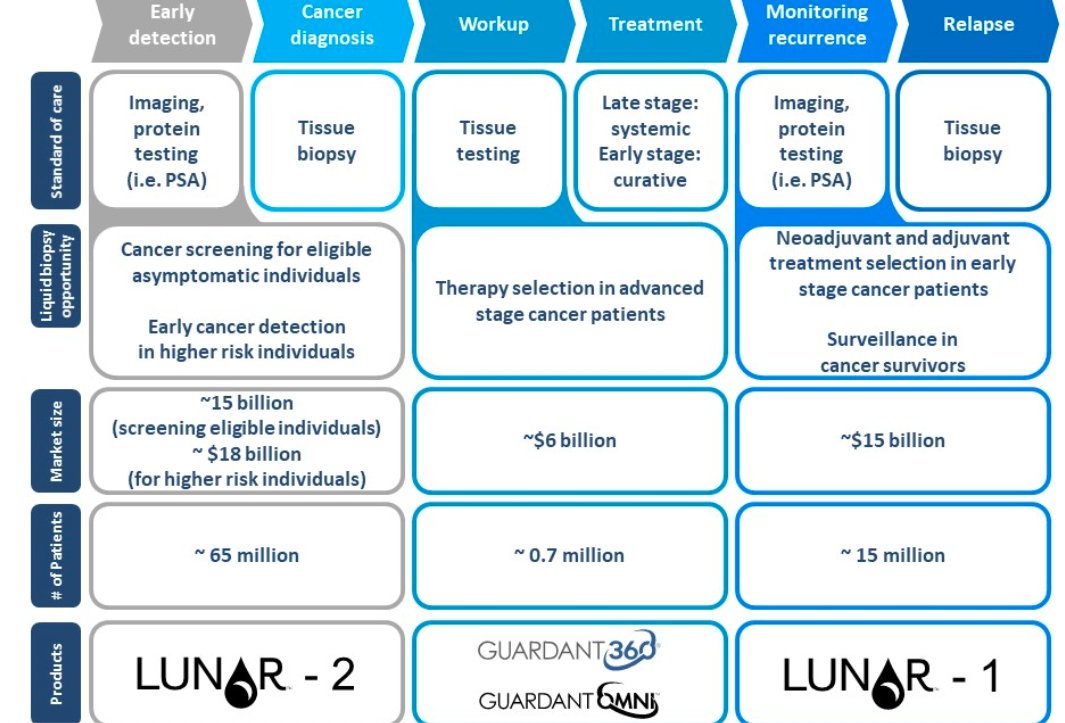
6/ Guardant Health (GH) has two commercial products, Guardant360 (the world& #39;s leading liquid biopsy) and GuardantOMNI for biopharma customers. And two more pipeline products (LUNAR 1 and 2)
Currently, most of the testing is done for NSCLC (non-small cell lung cancer).
Currently, most of the testing is done for NSCLC (non-small cell lung cancer).
7/ Another advantage is that biopharma customers can easily do more testing to see whether their gene therapies are working and if the ctDNA is now less concentrated. That& #39;s not really feasible with tissue biopsies.
8/ While Guardant is approved for NSCLC, it is currently trying to use its test for other types of cancer.
To be clear, liquid biopsies won& #39;t completely replace tissue biopsies yet. However, there will likely be a "liquid-first paradigm."
If positive, then no need for tissue.
To be clear, liquid biopsies won& #39;t completely replace tissue biopsies yet. However, there will likely be a "liquid-first paradigm."
If positive, then no need for tissue.
9/ But if negative through the liquid biopsy, there will likely be a tissue biopsy to ensure the diagnosis.
That& #39;s not to say liquid biopsies aren& #39;t accurate. They are. It& #39;s just that very early stage cancer can have very low concentrations of ctDNA.
That& #39;s not to say liquid biopsies aren& #39;t accurate. They are. It& #39;s just that very early stage cancer can have very low concentrations of ctDNA.
10/ Besides Guardant, Roche& #39;s Foundation Medicine has a diagnostic for prostate cancer or NSCLC patients who might benefit from immunotherapy.
https://www.aacc.org/cln/articles/2020/november/a-new-era-for-liquid-biopsy
I">https://www.aacc.org/cln/artic... wouldn& #39;t be surprised to see A LOT of new developments in this space in the next few years.
https://www.aacc.org/cln/articles/2020/november/a-new-era-for-liquid-biopsy
I">https://www.aacc.org/cln/artic... wouldn& #39;t be surprised to see A LOT of new developments in this space in the next few years.
End/ Natera is also working on a liquid biopsy as well as Illumina-owned GRAIL.
In other words, there is a lot of competition but the prize is huge.
Liquid biopsies will enable faster drug production through clinical trials and detect cancer much earlier.
In other words, there is a lot of competition but the prize is huge.
Liquid biopsies will enable faster drug production through clinical trials and detect cancer much earlier.

 Read on Twitter
Read on Twitter