I& #39;m excited to share this work with you by
@BK_SupraChem! She dug her teeth into a supramolecular behavior that we& #39;d formerly brushed away as "it& #39;s just weird"... https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> https://pubs.acs.org/doi/10.1021/jacs.0c10486">https://pubs.acs.org/doi/10.10...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> https://pubs.acs.org/doi/10.1021/jacs.0c10486">https://pubs.acs.org/doi/10.10...
@BK_SupraChem! She dug her teeth into a supramolecular behavior that we& #39;d formerly brushed away as "it& #39;s just weird"...
We found a chemical reaction cycle that forms dissipative supramolecular fibers. But, when these fibers are present, the reaction cycle operates up to 5x faster. So, the reaction cycle produces assemblies, and the assemblies catalyze the reaction cycle!
Also, pretty!!! https://abs.twimg.com/emoji/v2/... draggable="false" alt="😍" title="Lächelndes Gesicht mit herzförmigen Augen" aria-label="Emoji: Lächelndes Gesicht mit herzförmigen Augen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😍" title="Lächelndes Gesicht mit herzförmigen Augen" aria-label="Emoji: Lächelndes Gesicht mit herzförmigen Augen">
Also, pretty!!!
Such reciprocal coupling is frequently found in biology but synthetic examples are rare. That& #39;s a shame because much of the sophisticated structure formation is regulated by such catalytic mechanisms. Think of microtubule patterns or actin treadmilling.
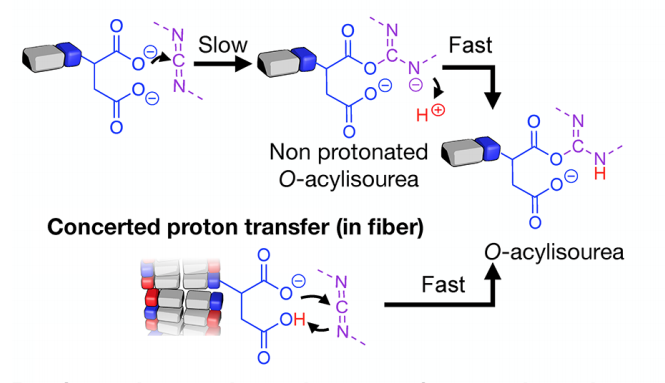
How does the reciprocal coupling work? It turns out that the assembly of molecules into fibers changes their local environment, which changes pKa-values and increases susceptibility to hydrolysis simultaneously. Even better, through molecular design, we can tune the effect!
What& #39;s next? The autocatalysis we found can lead to oscillations, pattern formation, and much more sophisticated behavior as found in biology. We hope to design such behavior in synthetic, non-equilibrium systems in the future.
Thanks to collaborators @TheKailaLab
for revealing the reaction pathways through calculations, and @hendrik_dietz for insights into the assembly through cryo-TEM.
Thanks to @emergenceoflife and @mattertolife for funding!
for revealing the reaction pathways through calculations, and @hendrik_dietz for insights into the assembly through cryo-TEM.
Thanks to @emergenceoflife and @mattertolife for funding!

 Read on Twitter
Read on Twitter