This is a great read on the nuance/trying to make sense of the remdesivir data! I highly respect @PaulSaxMD and his interpretation and clinical experience. This is how I want to feel about it too, but if I am being honest with myself the data do not really support it (1/7). https://twitter.com/PaulSaxMD/status/1317840625794940928">https://twitter.com/PaulSaxMD...
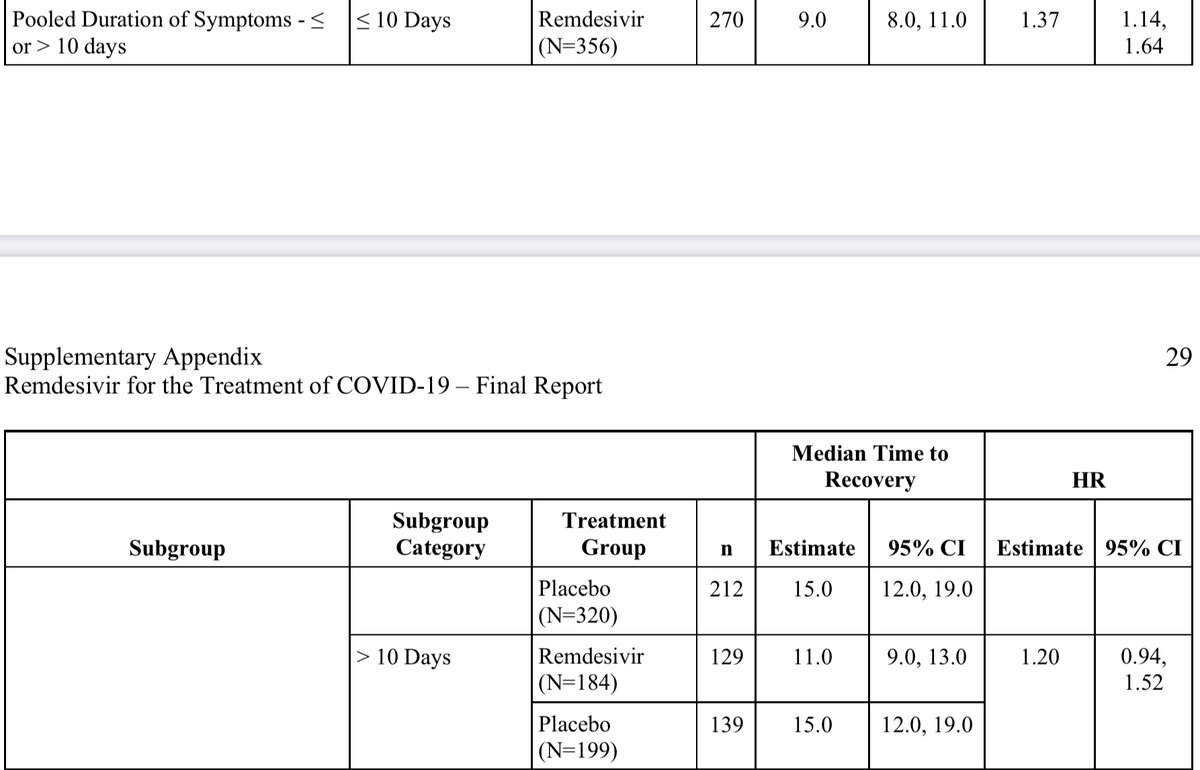
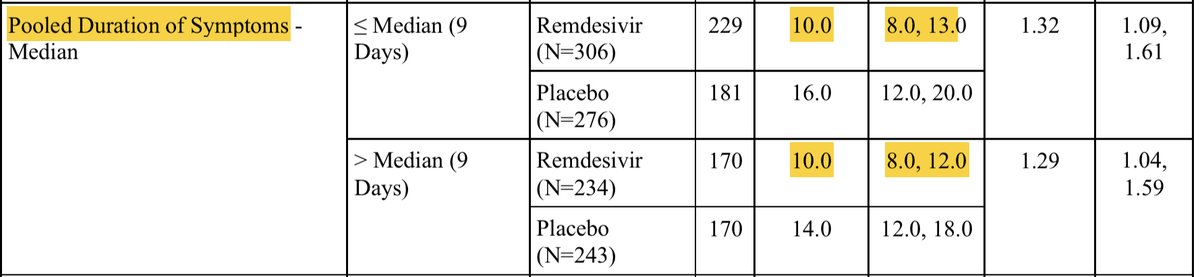
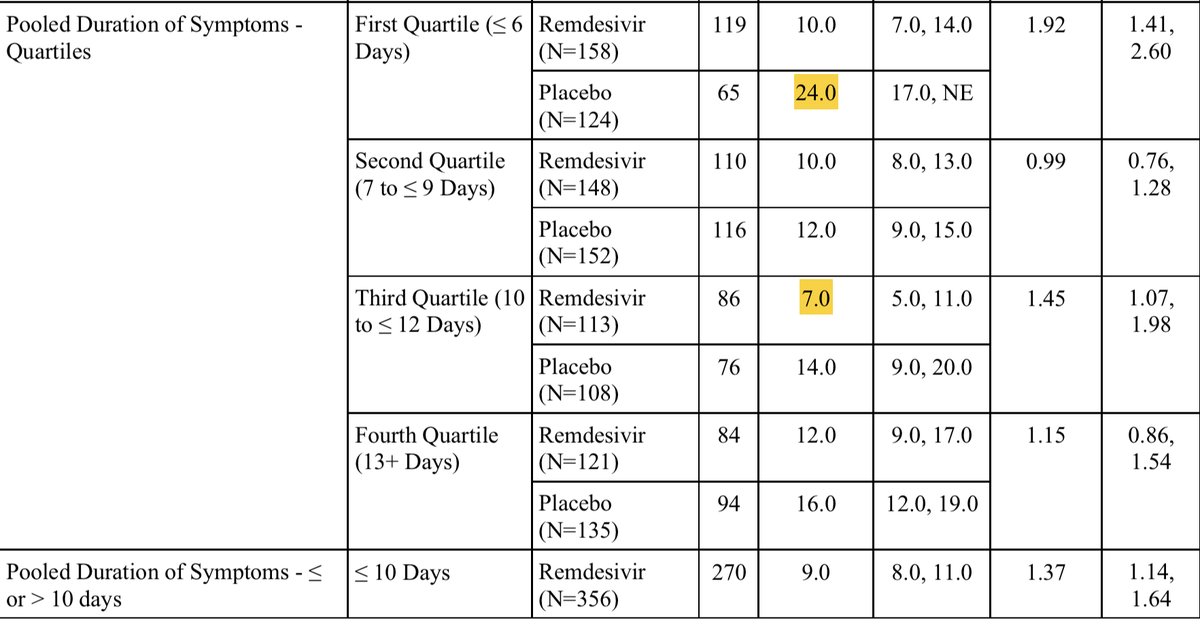
If time from onset of symptoms was the driver here, the difference seen in <=10 days, shouldn’t go away when we split it at 9 days. In fact, the shortest time to recovery was driven by the group days 10-12 From onset (<=6 days was no better). From supplement of ACTT-1
So those ten day splits are literally driven by patients presenting exactly on day 10 only. I struggle to see that as suggesting time from onset is meaningfully impacting any of the data we have to date.
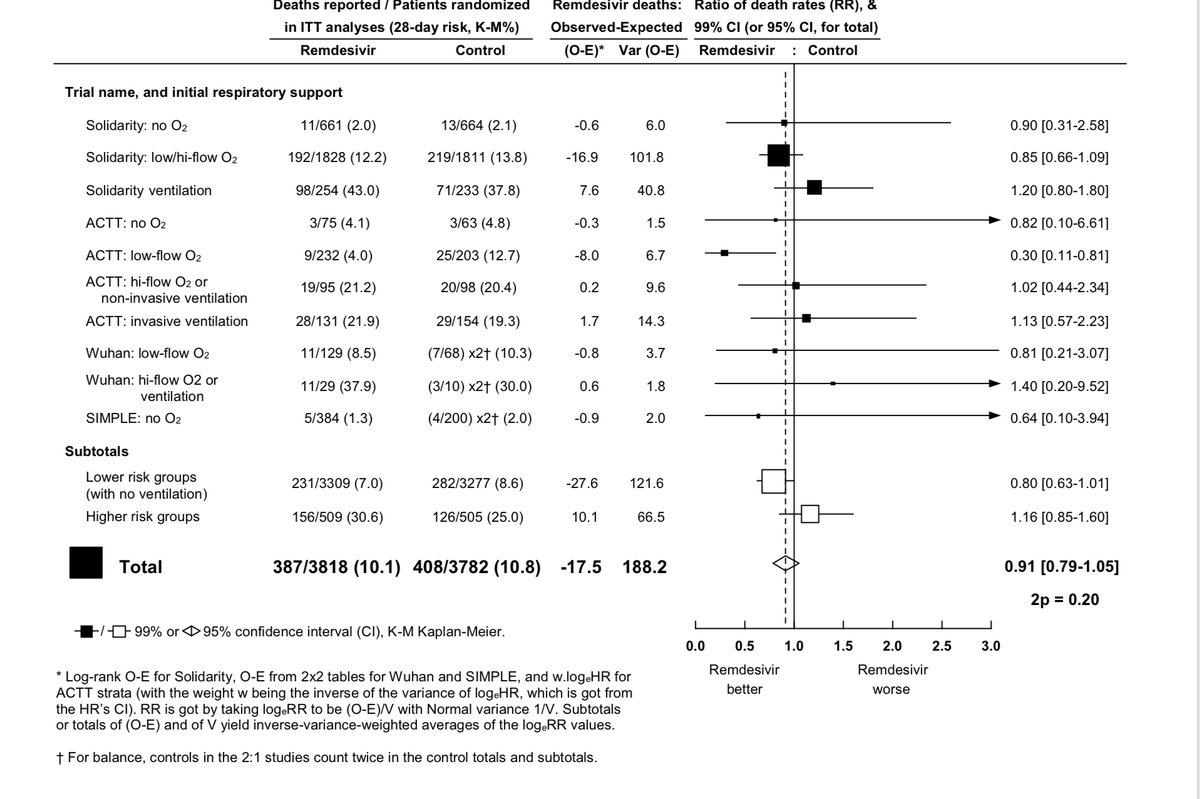
Finally, I do not think we can claim that the data suggest a mortality benefit in non-ventilated patients, but then write off the same discordance in the direction of harm for ventilated patients as statistical noise. It is more likely that neither are real.
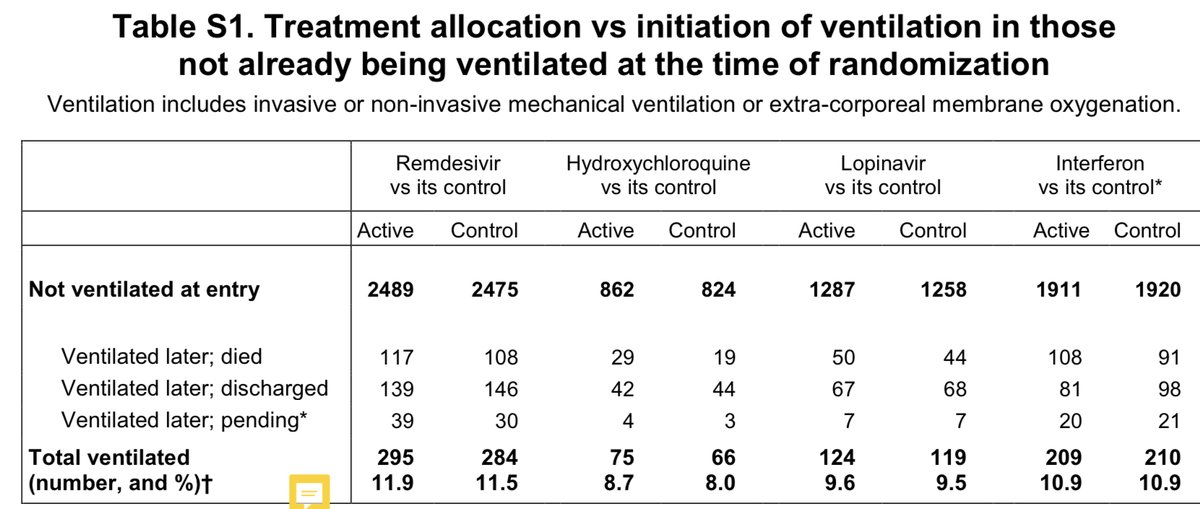
Remember, in solidarity there was also no improvement in progression to the need for ventilatory support in those not on it at baseline. This again suggests against a benefit in non-ventilated patients.
I do agree with the “probably (possibly) a modest benefit in some patients”, but do not think it is in the vicinity of the magnitude of the top line of ACTT-1. The other two trials (Wuhan RCT, Solidarity) suggest no impact on length of stay or progression of illness.
If we believe that there is a target population then I am very supportive of additional trials in that population - but even the most favorable view of the data as a whole suggests that there is not so large of a benefit that we can‘t study it first before using it there.

 Read on Twitter
Read on Twitter