Trial negative for all 4 tested interventions (HCQ, remdesivir, inferferon, lopinavir) but the reports terribly lacks granularity
Repurposed antiviral drugs for #COVID19; interim WHO SOLIDARITY trial results https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1">https://www.medrxiv.org/content/1...
Repurposed antiviral drugs for #COVID19; interim WHO SOLIDARITY trial results https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1">https://www.medrxiv.org/content/1...
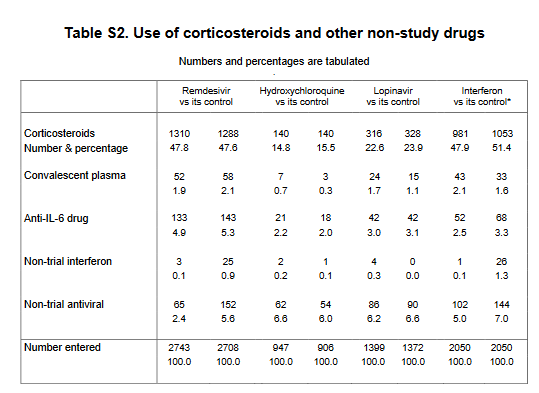
(eg this is why there was initially the DISCOVERY study). This was known from the start, & the focus of this joint effort coordinated by the CHO was on reducing mortality, which was legitimate, common for several countries. However, as one can see, the population was diverse, not
very well defined (the WHO has an ordinal scale and we don& #39;t even have a report of status on this ordinal scale - no difference between low-flow and high flow O2), the trial pop is overweighted in "Asia+Africa", etc. One may also ask what about the data by region, by subgrps
(with more granularity for the "on O2 at entry"). One way even wonder if the data varied along time (1st 4-6mo vs after dpding on the region). Well, this study had limitations. Still some surprising results, notably for the interferon group (no trend on non-ventilated pop)
whereas at least there is a numerical one for remdesivir on this group. Puzzling data, which probably invalidates the late use of all these interventions wrt the status on the ordinal scale past high-flow O2 supplementation, but for the earlier settings, the doors remain open.

 Read on Twitter
Read on Twitter