#EACTS2020 Update!
What a busy day! Great session related to TAVI and discussion of low risk patients and insights from the https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten"> TVT database containing results from over 330,000 patients! Read more below... warning, long but interesting (hopefully!) thread...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten"> TVT database containing results from over 330,000 patients! Read more below... warning, long but interesting (hopefully!) thread...
@EACTS @SCTSUK
What a busy day! Great session related to TAVI and discussion of low risk patients and insights from the
@EACTS @SCTSUK
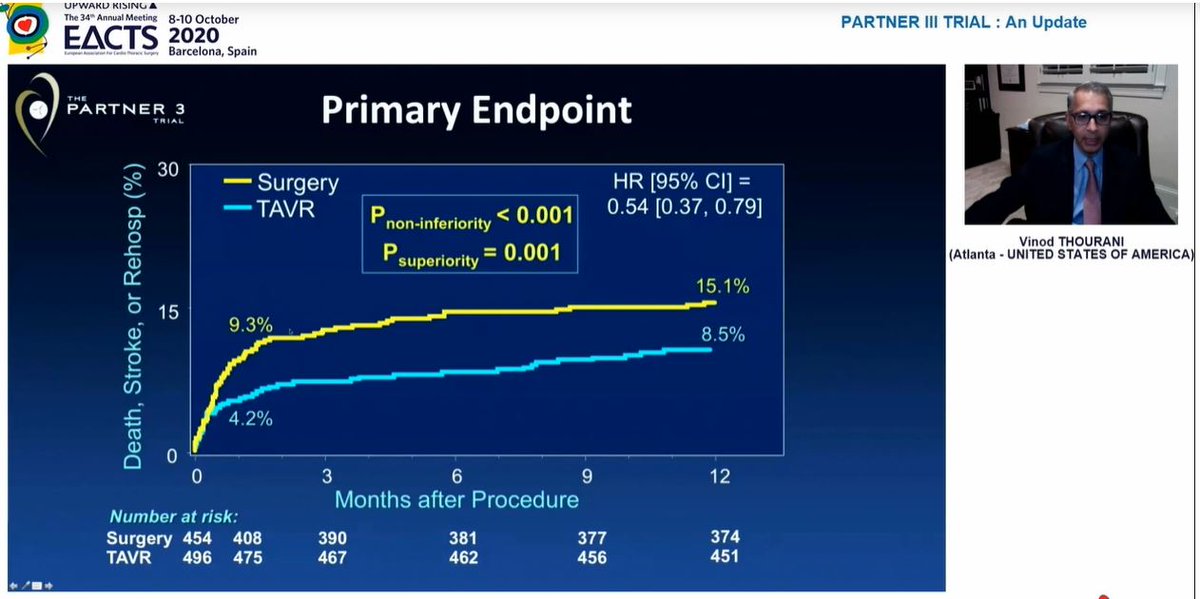
First presentation included 2yr outcomes from PARTNER 3 trial from Dr Vinod Thourani  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
We were reminded of the 1year results presented last year at ACC
Significant difference in composite 1o EP at 1yr, endpoint was death / stroke / rehospitalization
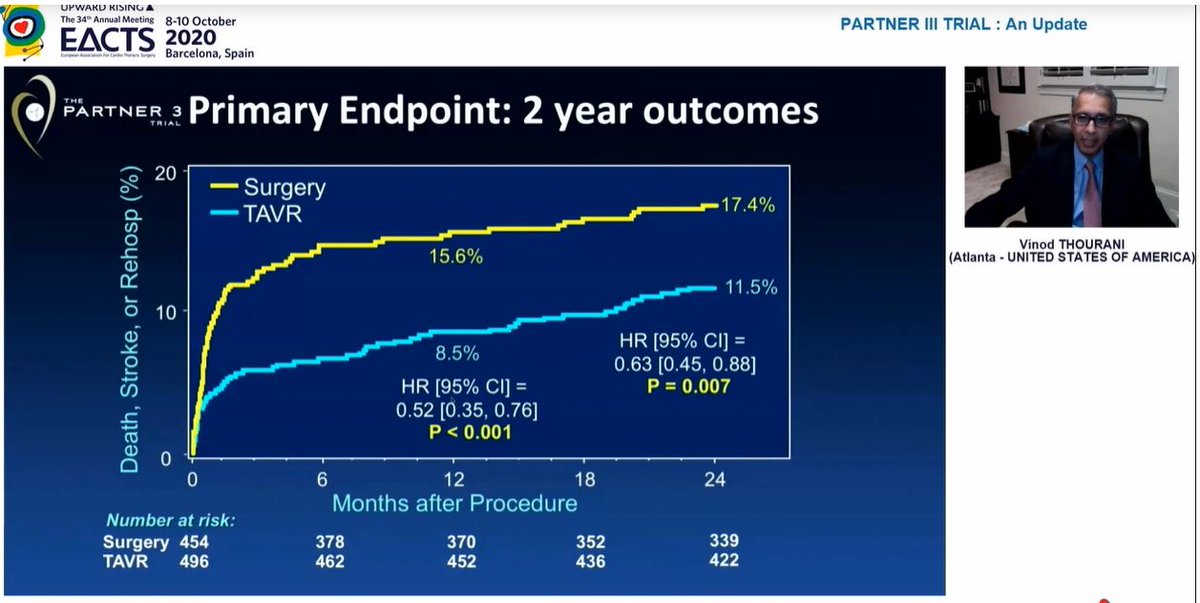
At 2 yrs, similar results for this composite EP
Significant difference in composite 1o EP at 1yr, endpoint was death / stroke / rehospitalization
At 2 yrs, similar results for this composite EP
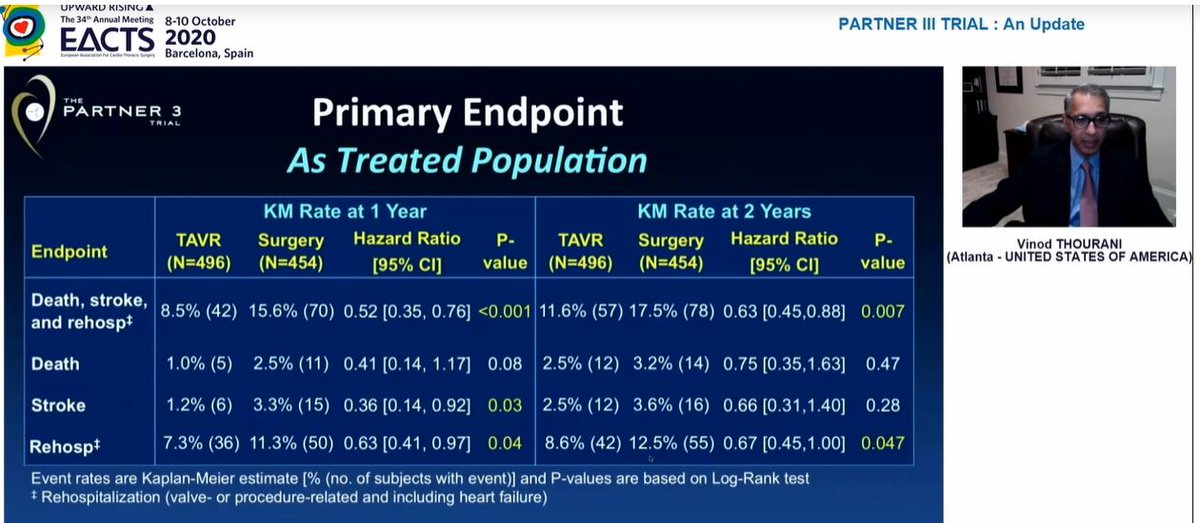
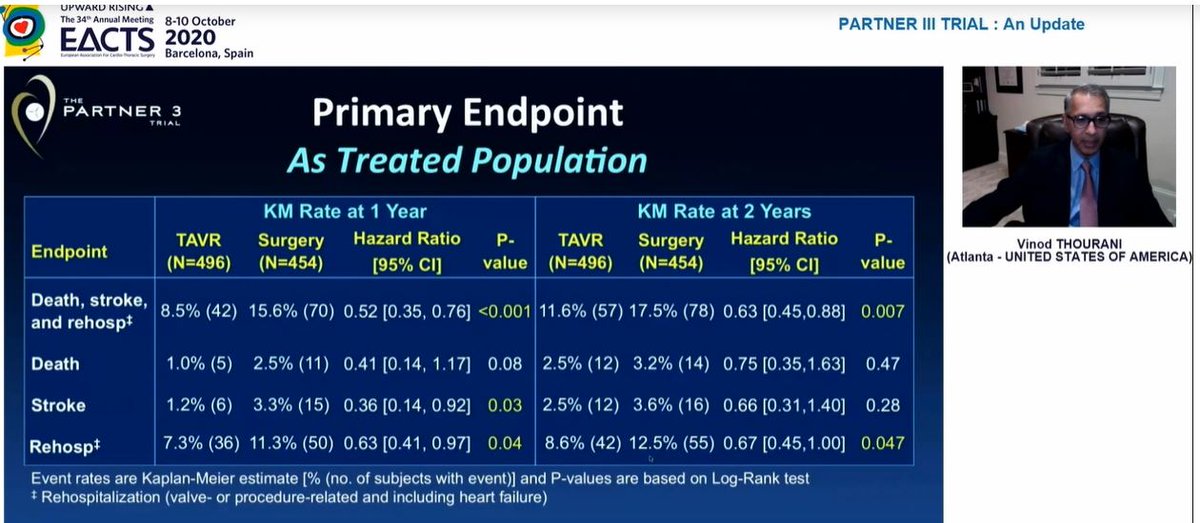
Some important observations from this table:
1) New LBBB (1 in 4) remains v.high with TAVI. This matters as data now shows new LBBB = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> survival
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> survival
2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice
3) Valve thrombosis https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs
1) New LBBB (1 in 4) remains v.high with TAVI. This matters as data now shows new LBBB =
2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice
3) Valve thrombosis
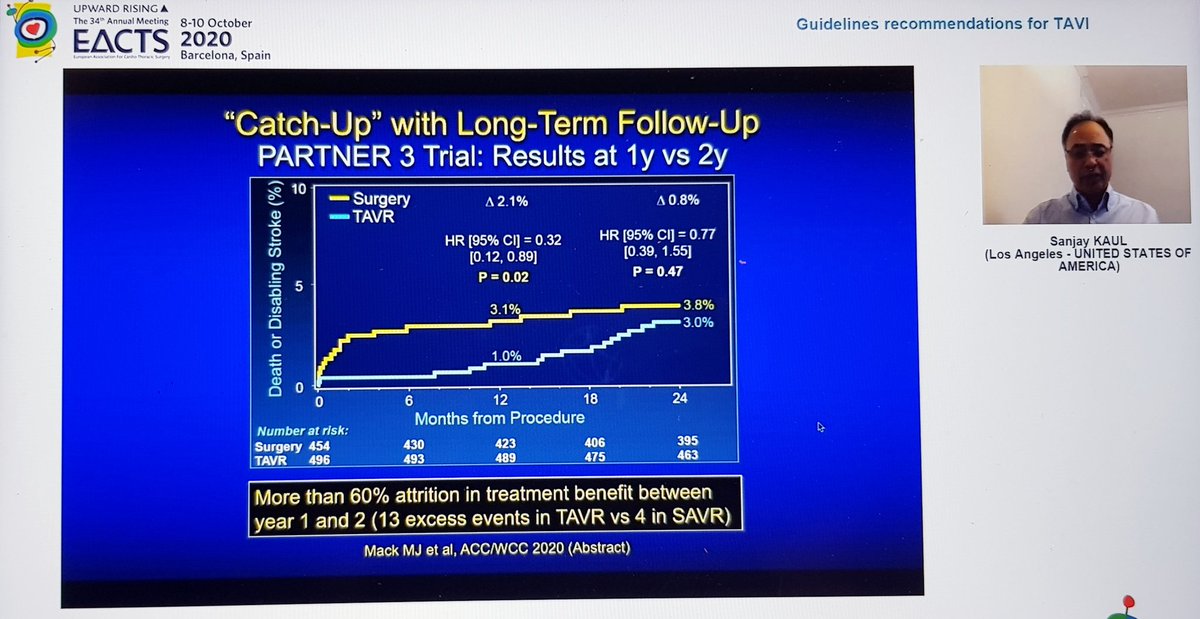
Also, the significant difference in stroke at 1yr had disappeared by 2 years, so significant difference in primary EP at 2yrs driven by rehospitalization
Conclusion - overall results favour TAVI at 2yrs but changes between years 1 and 2 clearly underline importance of longer term follow-up, which is planned until 10years - good to know!
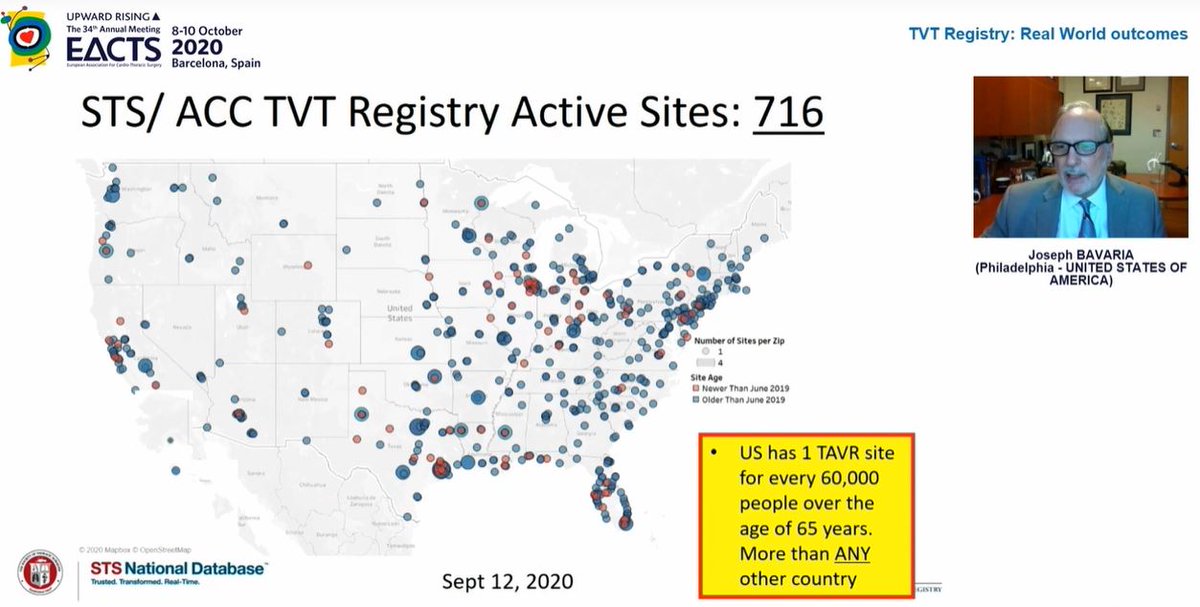
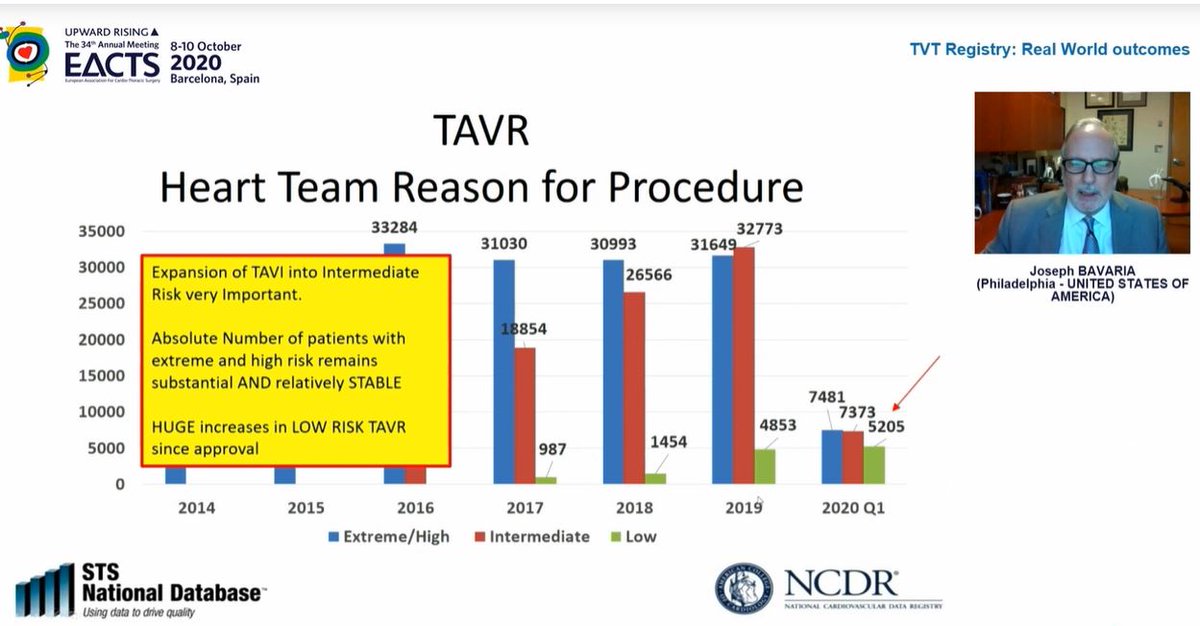
Next up, fascinating real world data from  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!
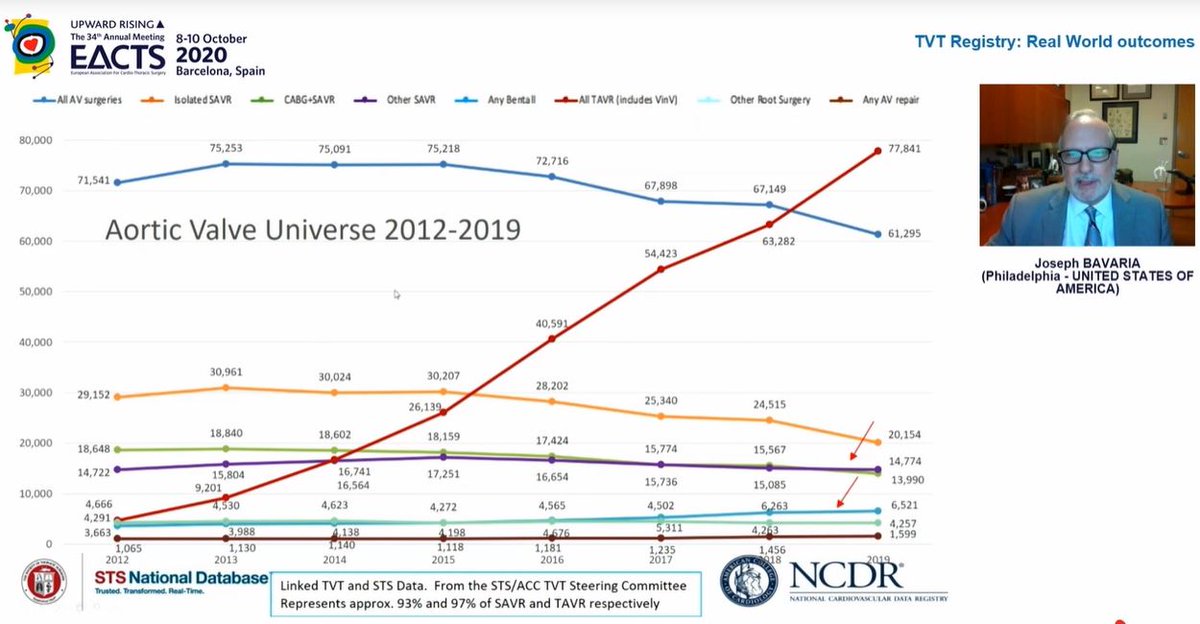
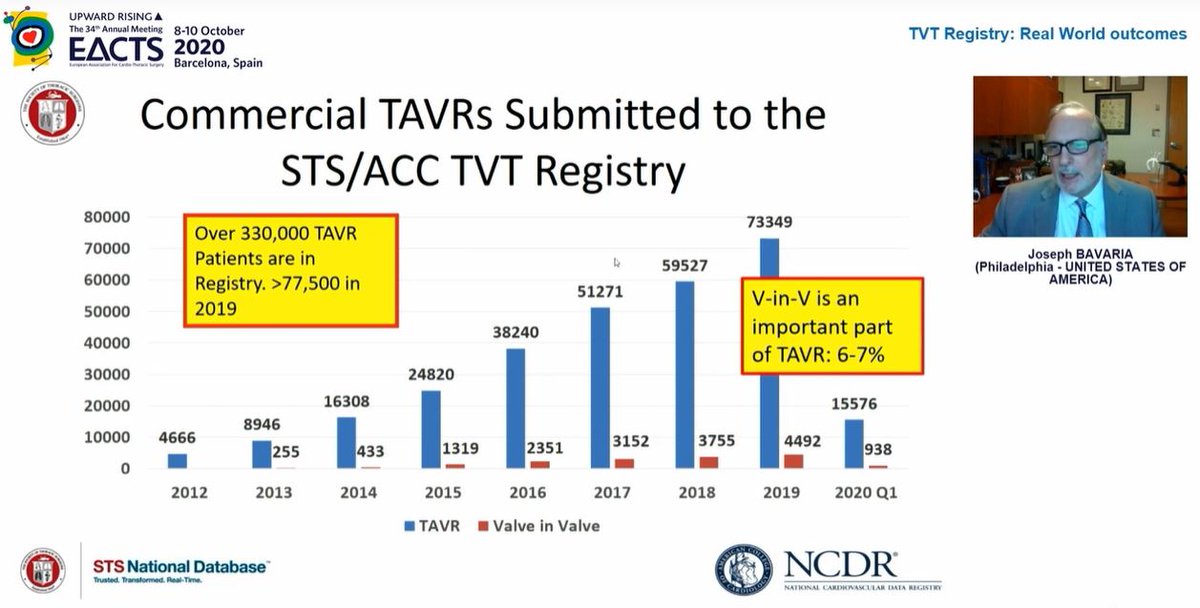
TAVI overtook isolated sAVR in 2016 in terms of volume but last year overtook sAVR for all procedures
Look at the effect of FDA approval for low risk patients on procedural numbers...in the FIRST QUARTER of 2020 alone, there were more low risk patients treated by TAVI than in the WHOLE of 2019! TAVI in low risk patients has increased massively...
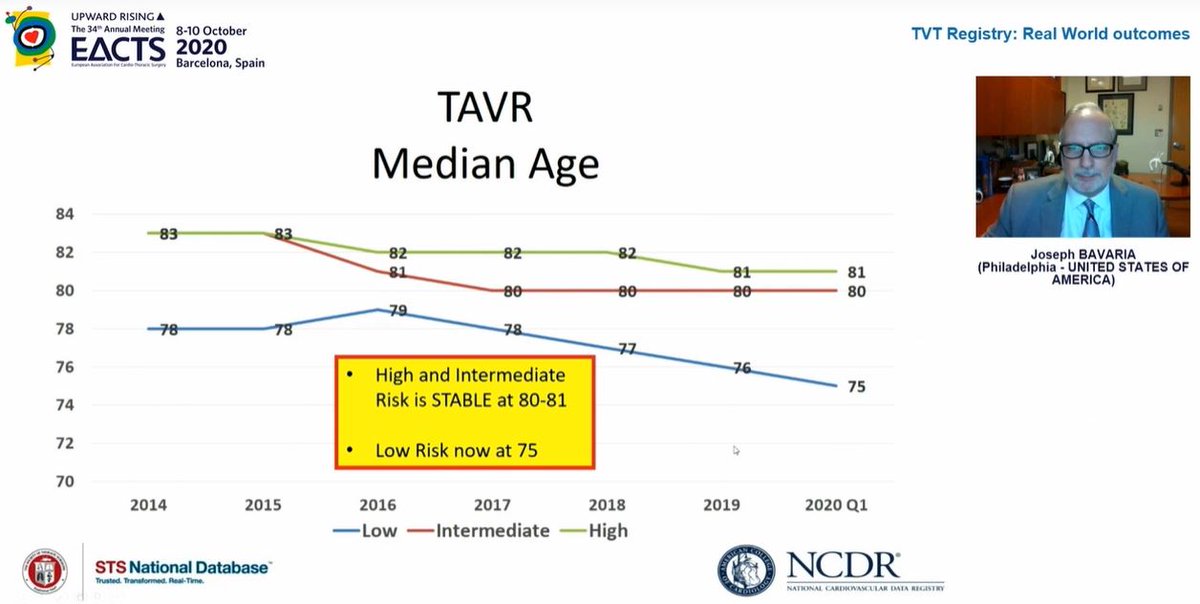
TAVI patient ages by surgical risk category
80-81 for high & intermediate risk
75 for low risk patients
80-81 for high & intermediate risk
75 for low risk patients
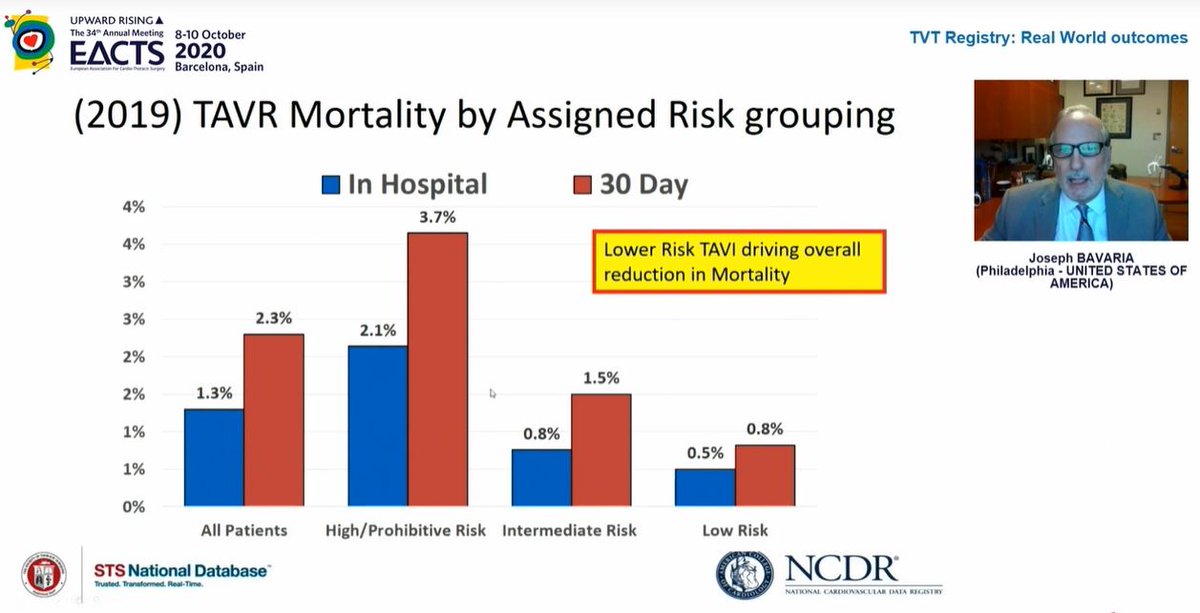
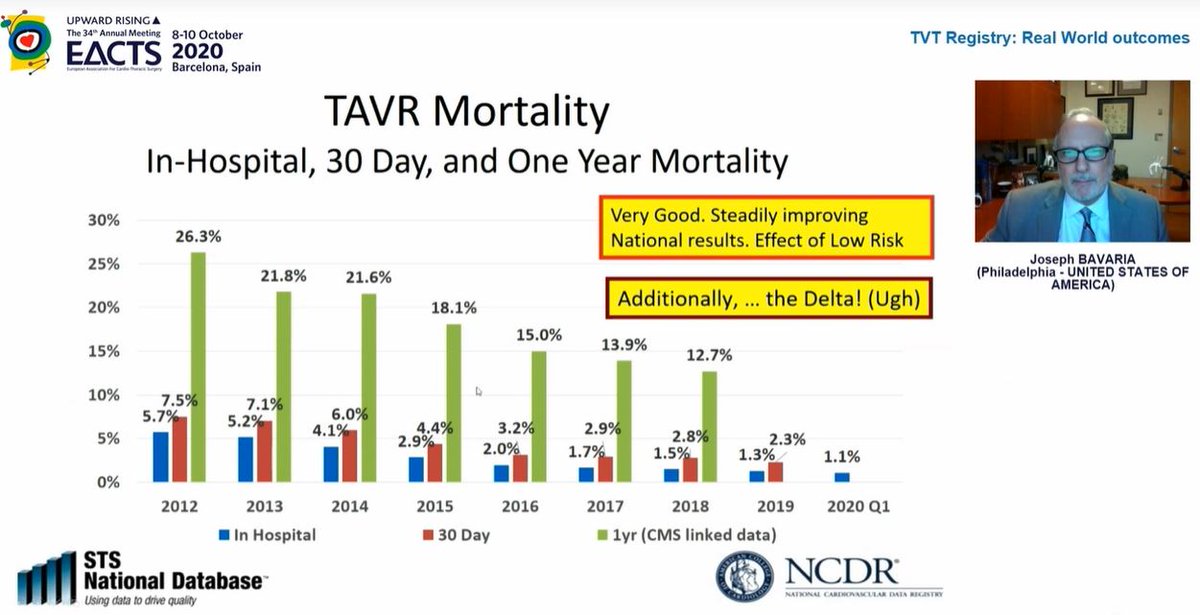
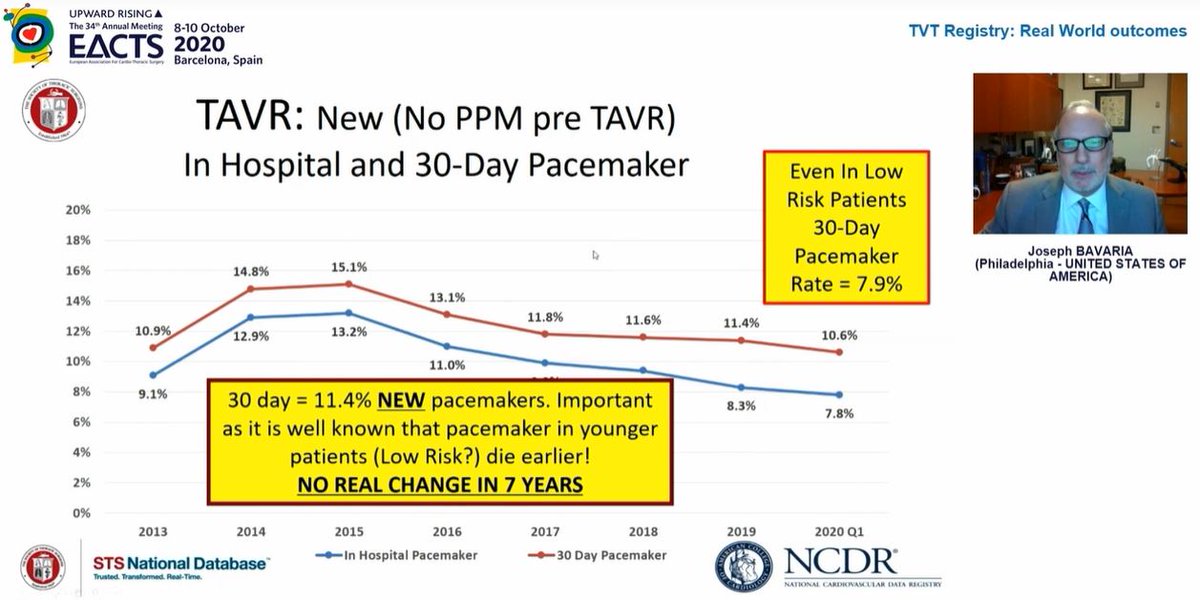
Mortality rates continue to fall which is hugely reassuring
Curiously, in 2nd image, look at 2019 data - in hospital mortality 1.3% but 30day mortality 2.3%! What is going on between day ~3 and day 30 such that death rate nearly *doubles*? This needs closer look to understand
Curiously, in 2nd image, look at 2019 data - in hospital mortality 1.3% but 30day mortality 2.3%! What is going on between day ~3 and day 30 such that death rate nearly *doubles*? This needs closer look to understand
Pacing rates haven& #39;t changed and remain higher than ideal...
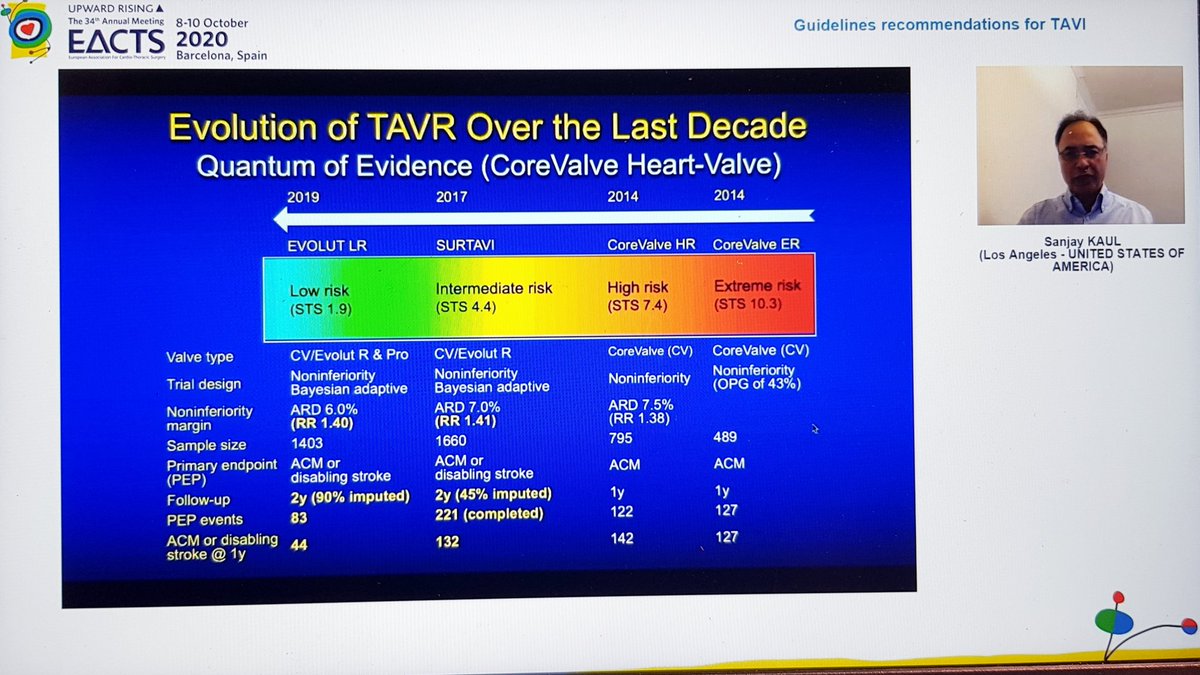
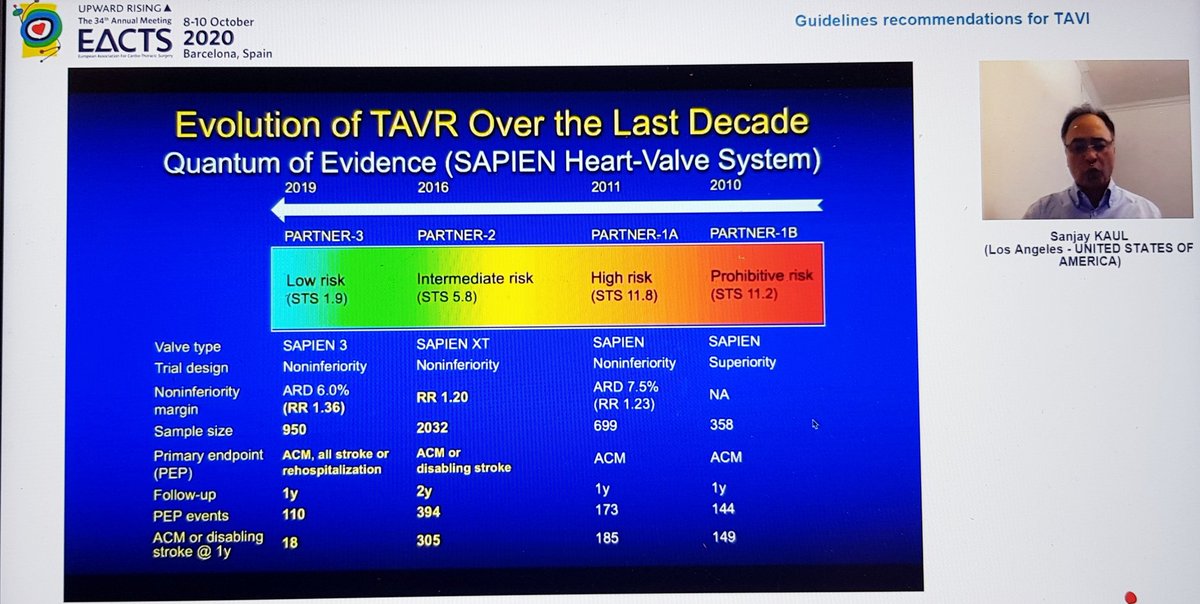
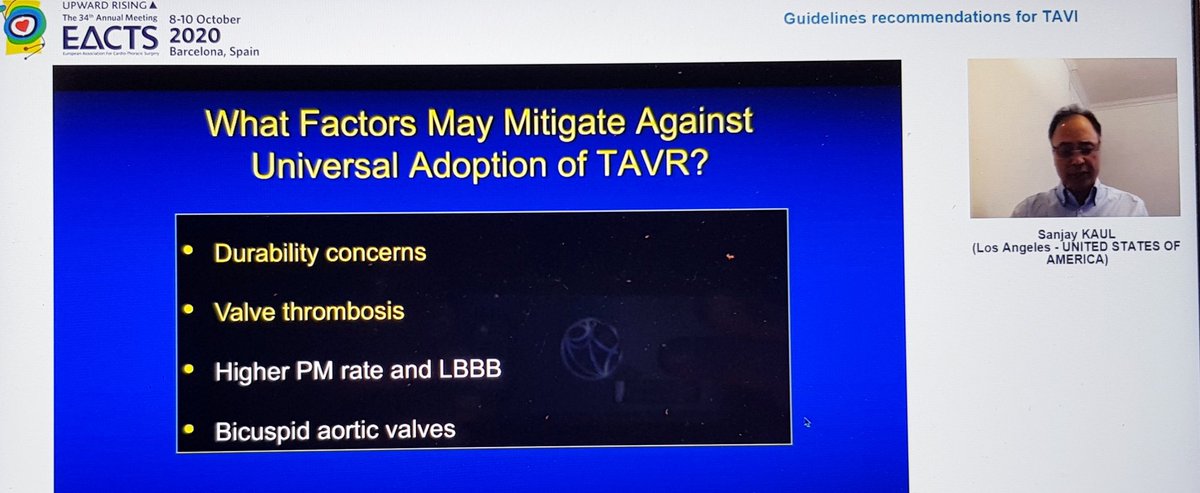
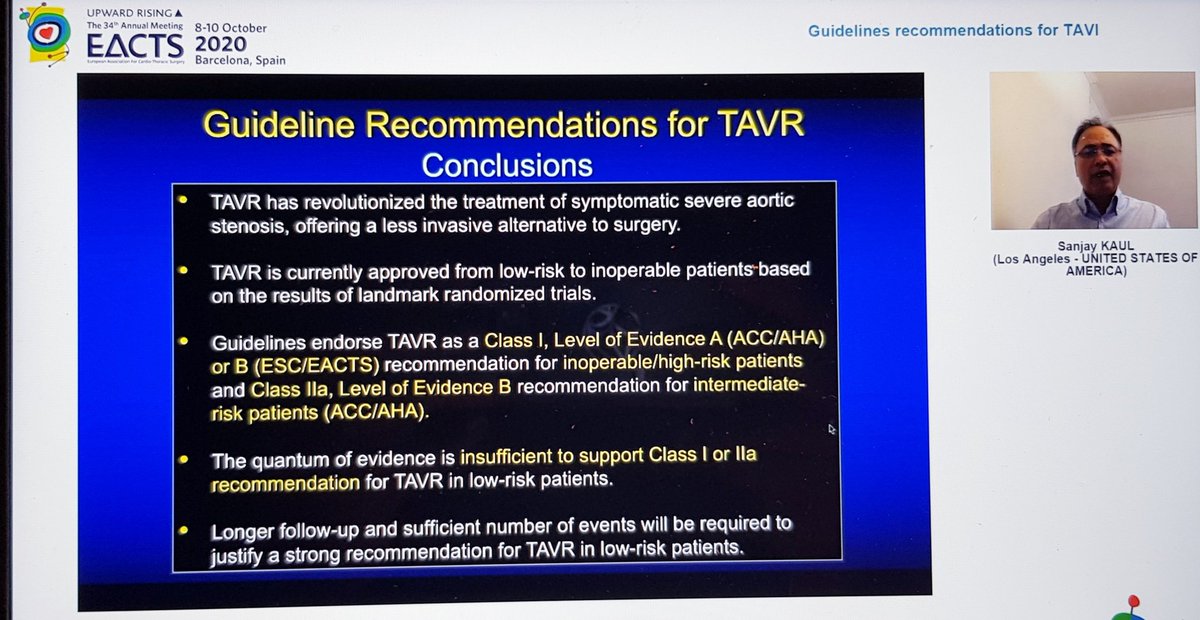
Finally, the Summary from Dr Kaul. Started with great slides highlighting trial strengths & weaknesses. For PARTNER 3, there& #39;s inclusion of rehospitalization in the 1o EP, which hadn& #39;t been in PARTNER 2. For CoreValve, only 10% pts completed 2yr F/U, 90% data imputed by modeling
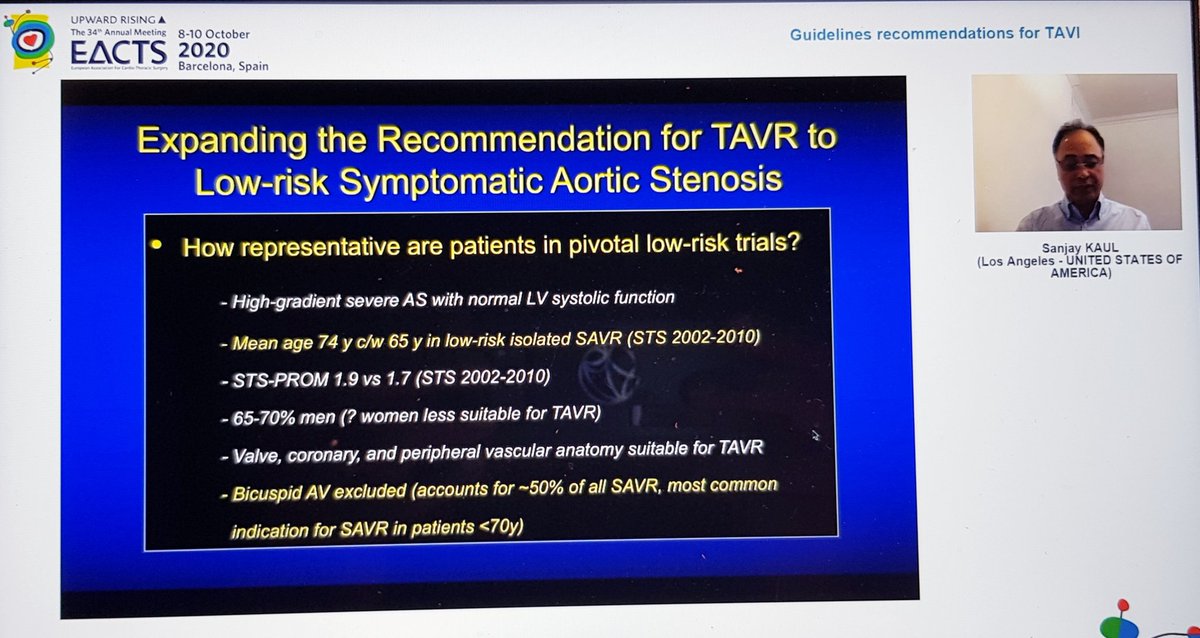
He reminded us of the rather over-enthusiastic (my words) Editorial last year stating that TAVI was now 1st line treatment in all patients...despite fact that bicuspid valve, younger patients, unfavourable anatomy etc all excluded!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">
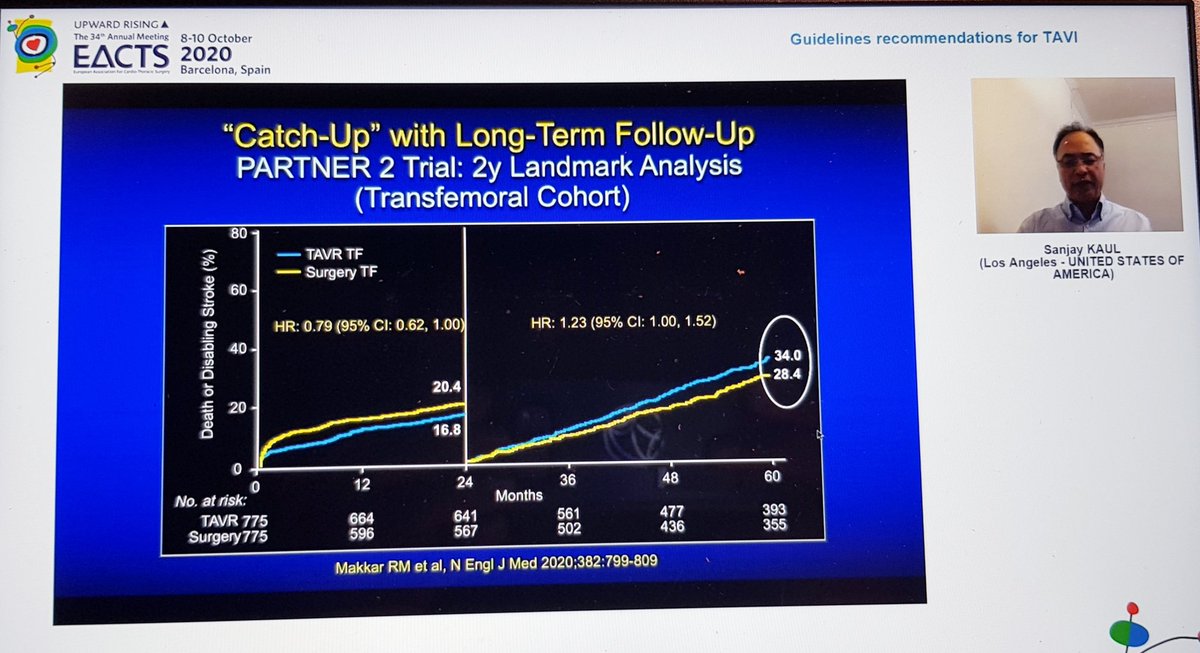
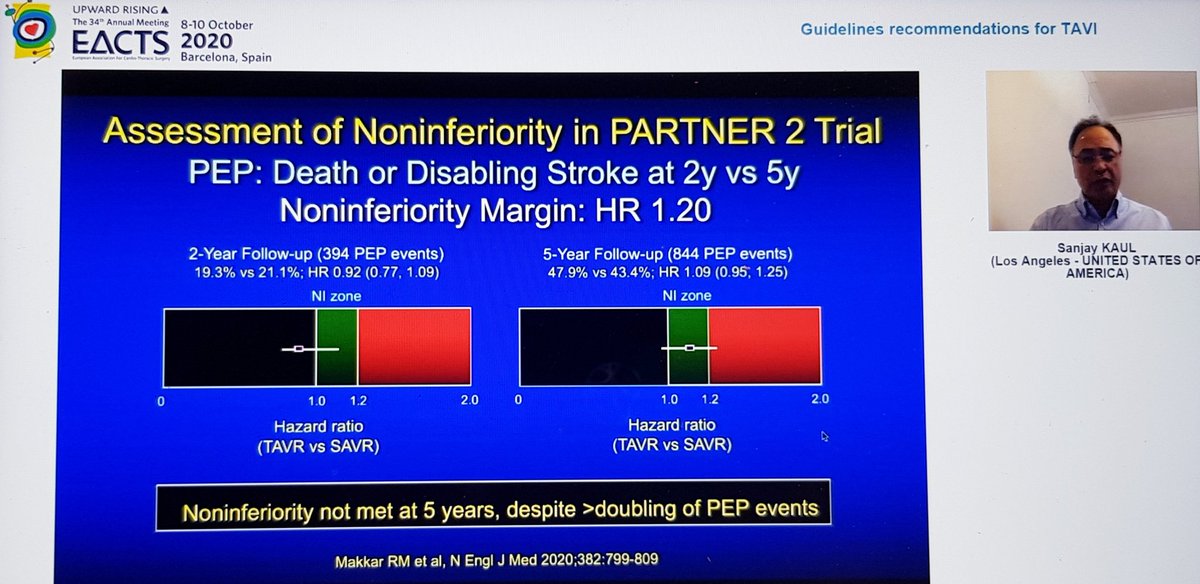
Importantly, he highlighted data from PARTNER 2 showing that although TAVI was non-inferior to sAVR at 2yrs, this was no longer true at 5yrs...i.e. if it& #39;s not non-inferior... then it& #39;s inferior
That& #39;s important, so I& #39;ll repeat - in PARTNER 2, TAVI no longer non-inferior at 5yrs
That& #39;s important, so I& #39;ll repeat - in PARTNER 2, TAVI no longer non-inferior at 5yrs
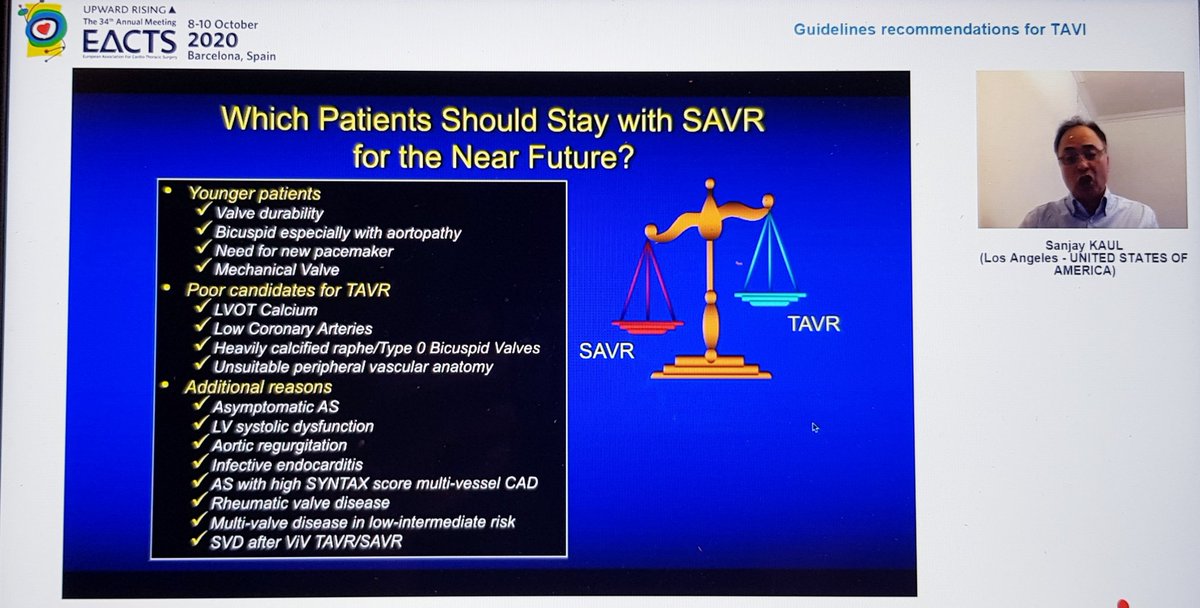
Great conclusions and a slide highlighting the (large) cohorts of patients for whom sAVR still appears to be the right choice
Followed by a panel discussion. By far my favourite part was when @drjohnm asked why PARTNER 3 included rehospitalization in 1o EP and the candid reply was (I& #39;m paraphrasing) "Well Edwards asked to include it & the FDA said sure, no problem"  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">
In conclusion, my thoughts:
-TAVI is a great innovation & superb example of successful Industry-clinician-scientist collaboration. It has allowed us to treat hundreds of thousands of patients that may otherwise have not had any treatment and it& #39;s arrival should be embraced
-TAVI is a great innovation & superb example of successful Industry-clinician-scientist collaboration. It has allowed us to treat hundreds of thousands of patients that may otherwise have not had any treatment and it& #39;s arrival should be embraced
I don& #39;t think anyone denies its prominent role for high risk severe AS patients and certainly an argument can be made for certain individuals at intermediate risk although 5yr data from PARTNER 2 suggest some caution would be prudent
Where biggest disagreements lie is with low risk. Many people, myself included, are surprised FDA approval was granted for a device designed to last a decade on the back of 1yr data. Pacemaker and new LBBB rates remain higher than we& #39;d like. Plus, 2yr data not as rosy as 1yr...
We really need regulators to insist on proper (clinically important) primary endpoints & imputing outcomes with modeling makes no sense when you could have just followed them up...
Finally, are we happy that companies making the valves are not just funding the pivotal Phase 3 trials of their own products, but now choose the participating centres, choose endpoints, collect the data and analyze the data?
Mitra-FR has shown it doesn& #39;t have to be this way...
Mitra-FR has shown it doesn& #39;t have to be this way...
I have no doubt one day TAVI will be the primary modality for most severe AS patients, but I don& #39;t think we are quite at the point of making it first line in all (or even most) low risk patients...yet
@SABOURETCardio @pomyers @M_Pompeu_Sa_MD @dompagano @mariovar55 @MMarinCuartas
@SABOURETCardio @pomyers @M_Pompeu_Sa_MD @dompagano @mariovar55 @MMarinCuartas

 Read on Twitter
Read on Twitter " title="First presentation included 2yr outcomes from PARTNER 3 trial from Dr Vinod Thourani https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">" class="img-responsive" style="max-width:100%;"/>
" title="First presentation included 2yr outcomes from PARTNER 3 trial from Dr Vinod Thourani https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">" class="img-responsive" style="max-width:100%;"/>
 survival2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice3) Valve thrombosis https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs" title="Some important observations from this table:1) New LBBB (1 in 4) remains v.high with TAVI. This matters as data now shows new LBBB = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> survival2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice3) Valve thrombosis https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs" class="img-responsive" style="max-width:100%;"/>
survival2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice3) Valve thrombosis https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs" title="Some important observations from this table:1) New LBBB (1 in 4) remains v.high with TAVI. This matters as data now shows new LBBB = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> survival2) New AF after sAVR is extraordinarily high(40%)! I don& #39;t see this in my daily practice3) Valve thrombosis https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> with TAVI at 2 yrs" class="img-responsive" style="max-width:100%;"/>

 . 716 TAVI sites across the country!" title="Next up, fascinating real world data from https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!">
. 716 TAVI sites across the country!" title="Next up, fascinating real world data from https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!">
 . 716 TAVI sites across the country!" title="Next up, fascinating real world data from https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!">
. 716 TAVI sites across the country!" title="Next up, fascinating real world data from https://abs.twimg.com/emoji/v2/... draggable="false" alt="🇺🇸" title="Flagge der Vereinigten Staaten" aria-label="Emoji: Flagge der Vereinigten Staaten">. 716 TAVI sites across the country!">
 " title="He reminded us of the rather over-enthusiastic (my words) Editorial last year stating that TAVI was now 1st line treatment in all patients...despite fact that bicuspid valve, younger patients, unfavourable anatomy etc all excluded! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">">
" title="He reminded us of the rather over-enthusiastic (my words) Editorial last year stating that TAVI was now 1st line treatment in all patients...despite fact that bicuspid valve, younger patients, unfavourable anatomy etc all excluded! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">">
 " title="He reminded us of the rather over-enthusiastic (my words) Editorial last year stating that TAVI was now 1st line treatment in all patients...despite fact that bicuspid valve, younger patients, unfavourable anatomy etc all excluded! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">">
" title="He reminded us of the rather over-enthusiastic (my words) Editorial last year stating that TAVI was now 1st line treatment in all patients...despite fact that bicuspid valve, younger patients, unfavourable anatomy etc all excluded! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤦🏽♂️" title="Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)" aria-label="Emoji: Mann schlägt sich die Hand vors Gesicht (mittlerer Hautton)">">
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">" title="Followed by a panel discussion. By far my favourite part was when @drjohnm asked why PARTNER 3 included rehospitalization in 1o EP and the candid reply was (I& #39;m paraphrasing) "Well Edwards asked to include it & the FDA said sure, no problem" https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">" class="img-responsive" style="max-width:100%;"/>
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">" title="Followed by a panel discussion. By far my favourite part was when @drjohnm asked why PARTNER 3 included rehospitalization in 1o EP and the candid reply was (I& #39;m paraphrasing) "Well Edwards asked to include it & the FDA said sure, no problem" https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">" class="img-responsive" style="max-width:100%;"/>


