Some comments on treating w/ the @Regeneron monoclonal antibody infusion.
1. @statnews piece by @matthewherper https://www.statnews.com/2020/09/29/regenerons-covid-19-antibody-may-help-non-hospitalized-patients-recover-faster-early-data-show/
and">https://www.statnews.com/2020/09/2... company press release with more extensive data
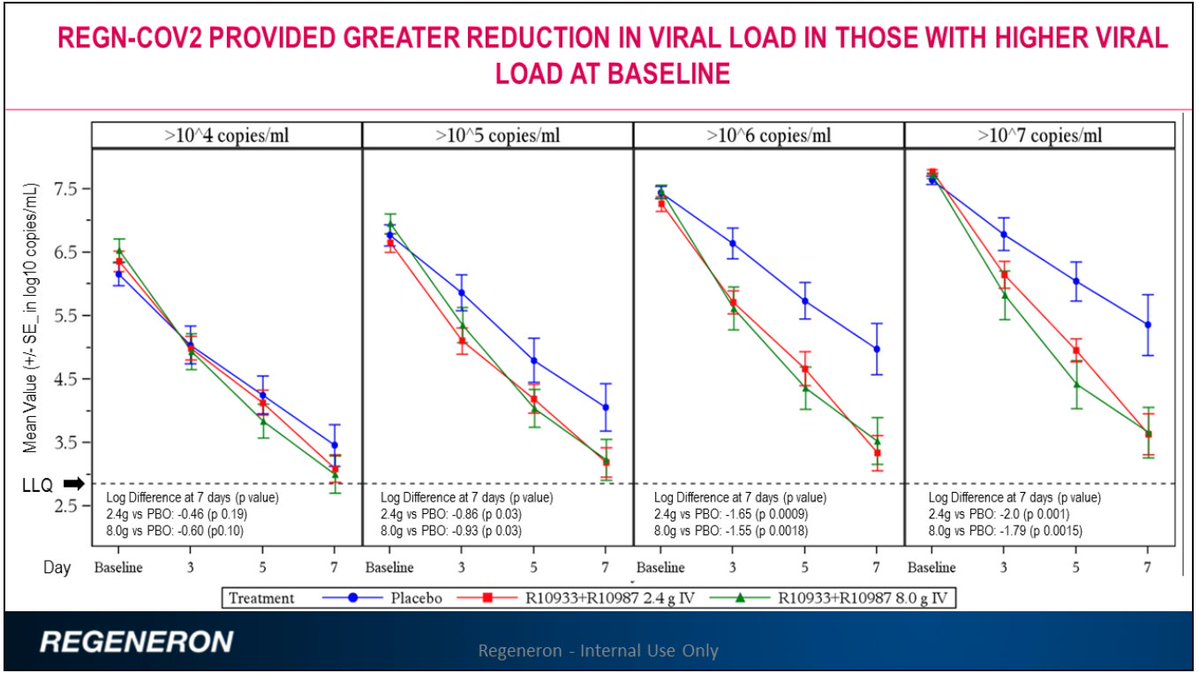
The viral load clearing effects were related to being antibody negative
1. @statnews piece by @matthewherper https://www.statnews.com/2020/09/29/regenerons-covid-19-antibody-may-help-non-hospitalized-patients-recover-faster-early-data-show/
and">https://www.statnews.com/2020/09/2... company press release with more extensive data
The viral load clearing effects were related to being antibody negative
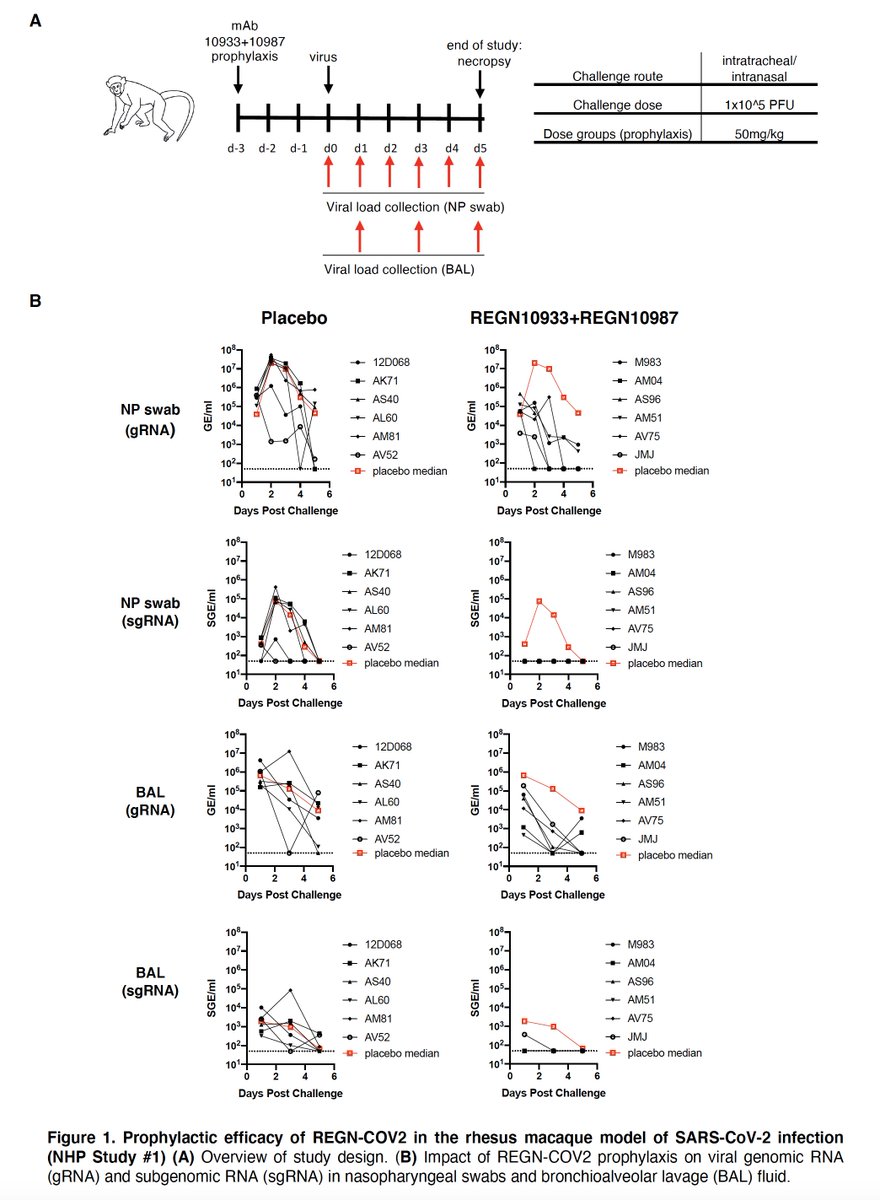
2. The dose given (8 g) was the highest tested. There were also non-human primate studies.
Here is the main paper @ScienceMagazine rationale for the "cocktail" as compared to a single monoclonal for the other antibody programs.
https://science.sciencemag.org/content/369/6506/1014">https://science.sciencemag.org/content/3...
Here is the main paper @ScienceMagazine rationale for the "cocktail" as compared to a single monoclonal for the other antibody programs.
https://science.sciencemag.org/content/369/6506/1014">https://science.sciencemag.org/content/3...
3. Here are the prophylaxis and treatment data preprint in macaques and hamsters
https://www.biorxiv.org/content/10.1101/2020.08.02.233320v1.full.pdf">https://www.biorxiv.org/content/1...
https://www.biorxiv.org/content/10.1101/2020.08.02.233320v1.full.pdf">https://www.biorxiv.org/content/1...
4. A balanced status check from a few days ago https://www.washingtonpost.com/health/2020/09/30/monoclonal-antibodies-to-treat-covid-19/">https://www.washingtonpost.com/health/20... by @Carolynyjohnson
5. That& #39;s the summary of the data set for this experimental therapy. So far safety has not been a stumbling block. But what isn& #39;t known is the indication for using it today as "precautionary." Still many unknowns about the mAbs, too.
6. BTW both companies ( @LillyPad and @Regeneron) with release of their small Phase 2 trials have asserted they have enough data to apply for an FDA emergency use authorization. I disagree, but that& #39;s a different matter.
7. @Regeneron& #39;s note today on their compassionate use program
https://investor.regeneron.com/static-files/fd58ba6a-f401-47b0-9d65-cfb01c313ec6
That& #39;s">https://investor.regeneron.com/static-fi... not really a clinical trial. It& #39;s an open use of drug w/o controls, provides some safety data. Would be interesting to learn how many patients received the mAb to date in this program.
https://investor.regeneron.com/static-files/fd58ba6a-f401-47b0-9d65-cfb01c313ec6
That& #39;s">https://investor.regeneron.com/static-fi... not really a clinical trial. It& #39;s an open use of drug w/o controls, provides some safety data. Would be interesting to learn how many patients received the mAb to date in this program.

 Read on Twitter
Read on Twitter