So, I see we are still doing this. Pretty amazing how selective and inconsistent this analysis is. Simply put, these data do not support the conclusions, and you cannot simply report event rate without assessing the details. Let’s look at this (BRIEFLY) and then please move on https://twitter.com/_MiguelHernan/status/1311304453484679174">https://twitter.com/_MiguelHe...
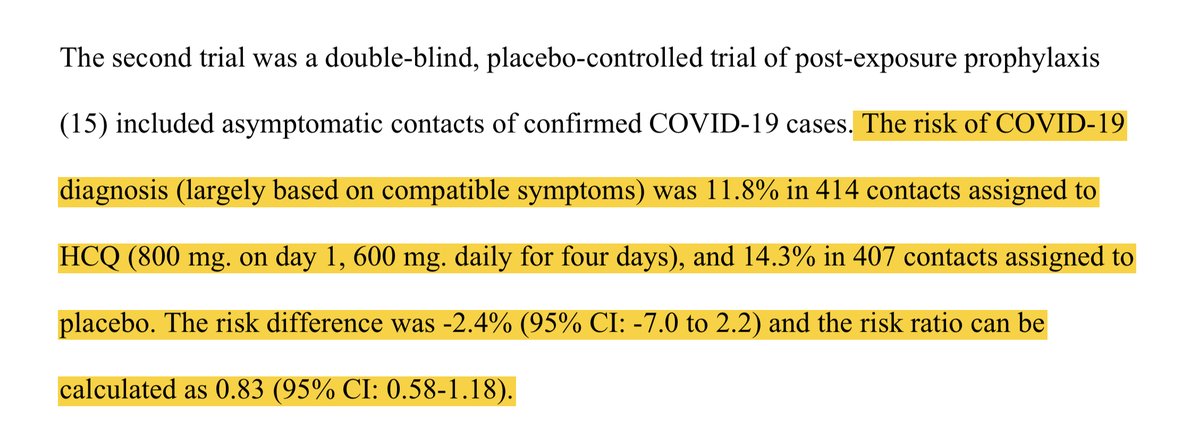
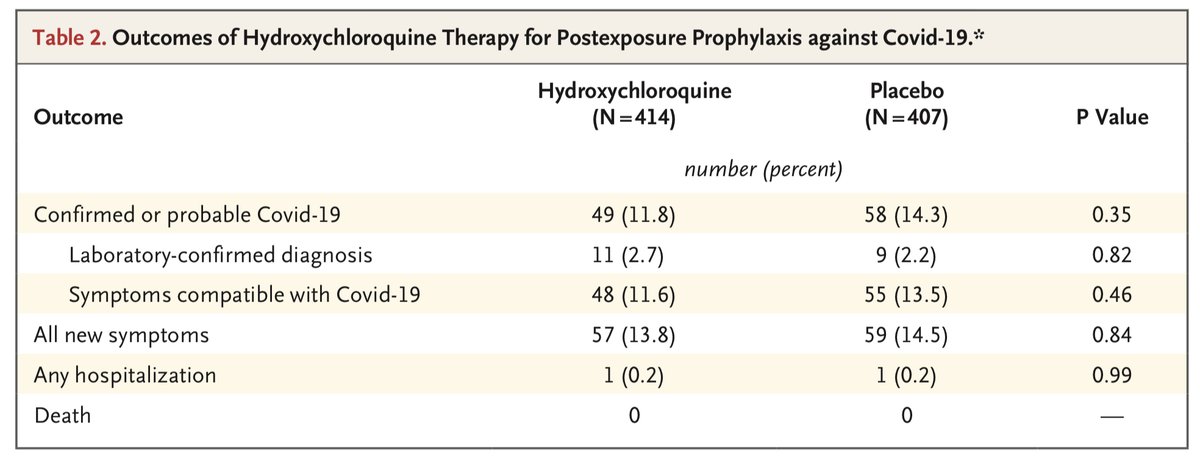
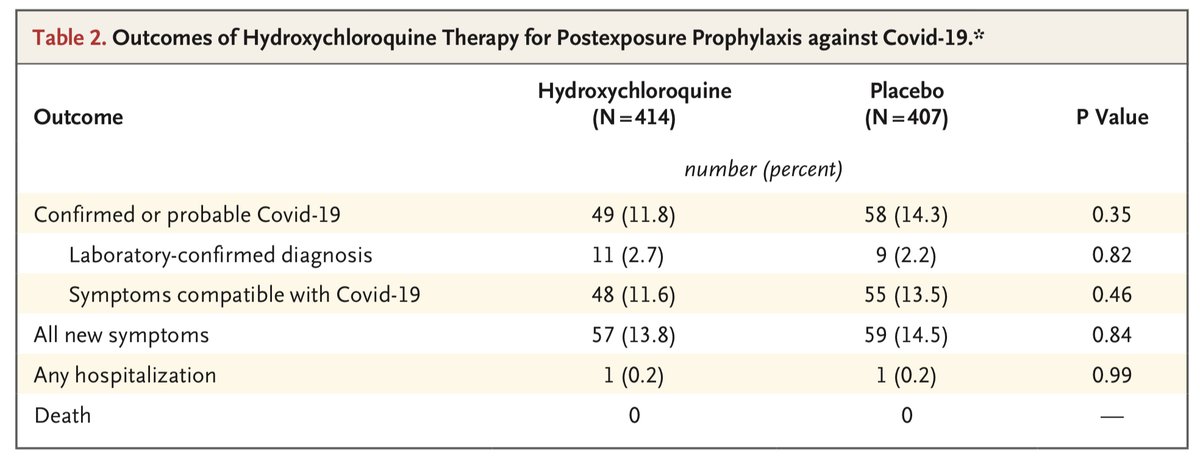
We will start w/ Boulware post-exposure prophy which is listed as an RR of 0.83 in the meta. As we have discussed before, the difference in PCR confirmed or probable SARS CoV-2 was completely driven by patients that did not receive HCQ. Thus, HCQ clearly didn’t impact this
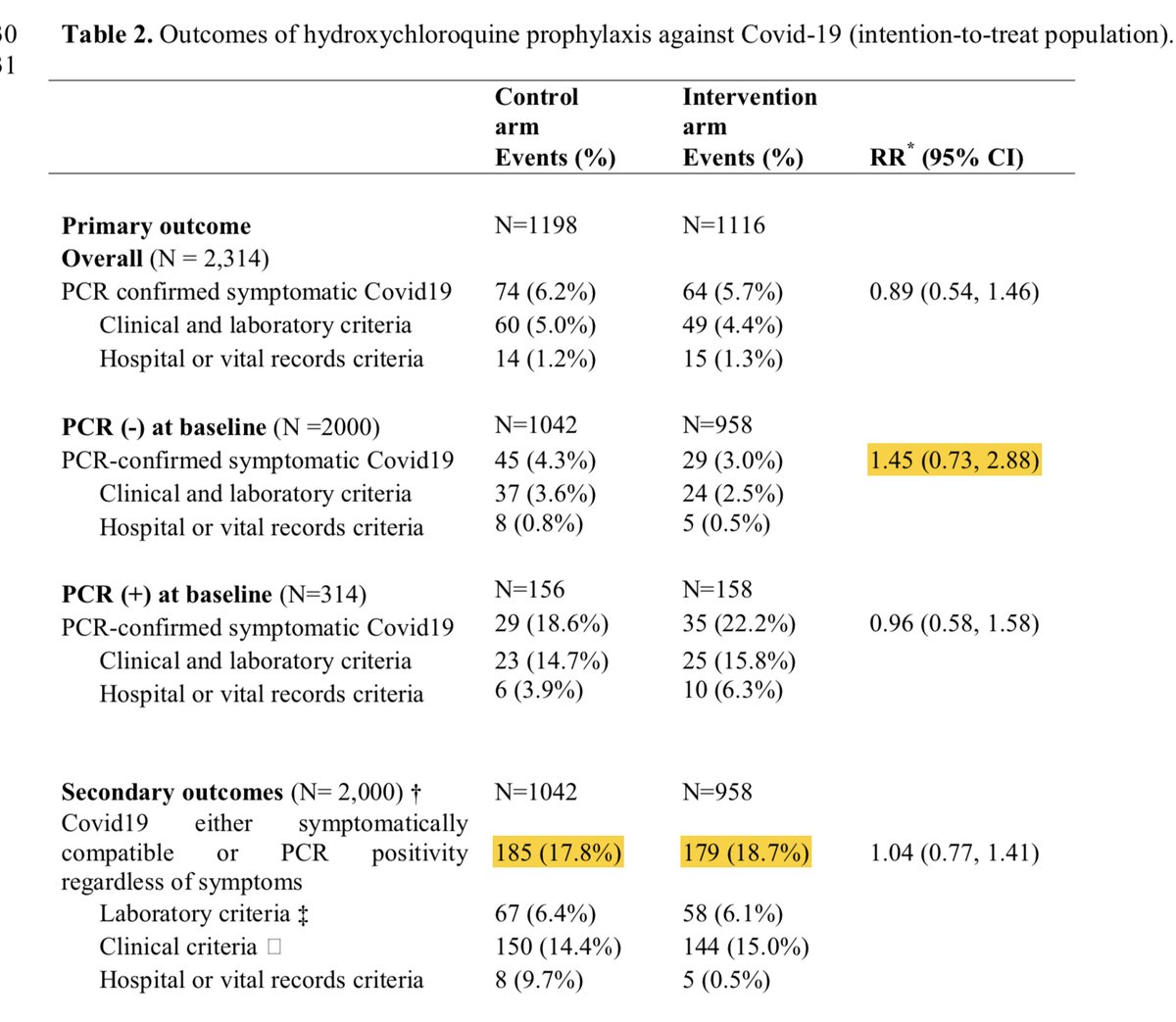
Second study - Mitjà. Interestingly in this one the authors of the meta focused on PCR confirmed disease as the outcome leading to a RR of 0.69. Interesting, since if they followed that criteria w/ Boulware there would have been 23% higher incidence w/ HCQ.
Similarly, had they chosen the same endpoint of PCR confirmed or probable cases you would have again seen inconsistent findings w/ 5% higher w/ HCQ in the Mitjà study. Also, keep in mind the difference here in Boulware was driven by patients who didn’t receive HCQ
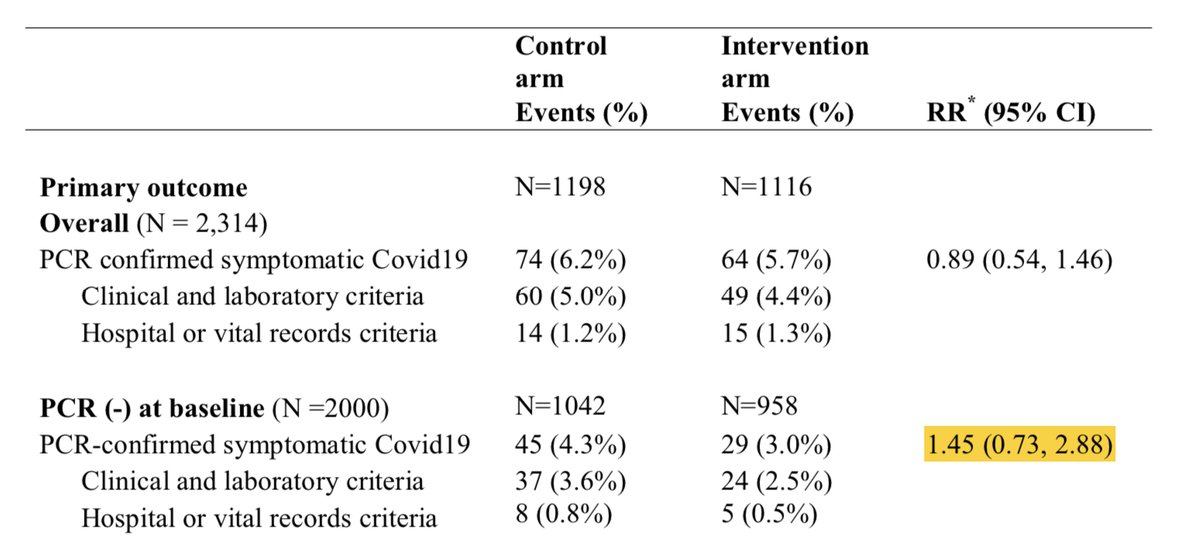
Finally, they discuss the latest trial by Rajasingham which, is a very different study design, with a different dose strategy, different duration, and degree of exposures. But whatever, let& #39;s ignore that for a second
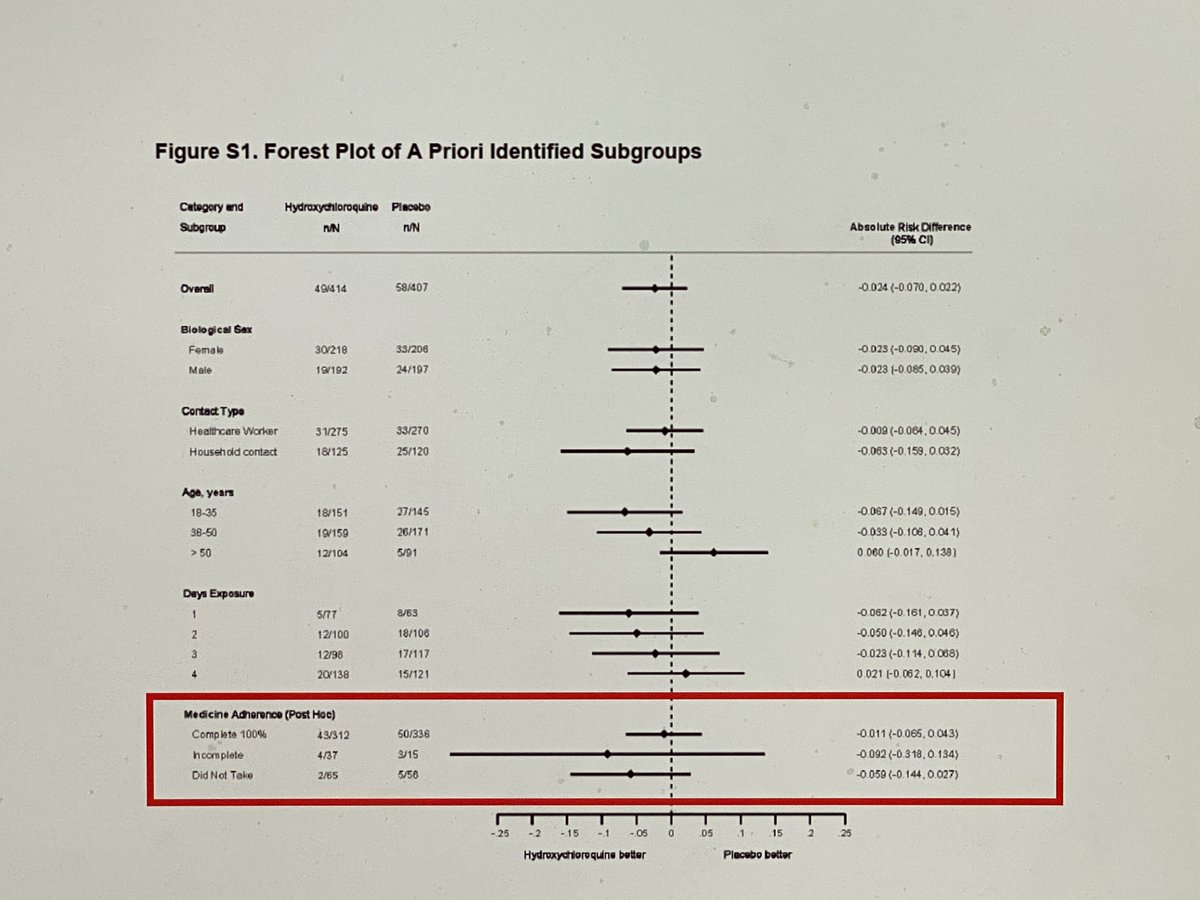
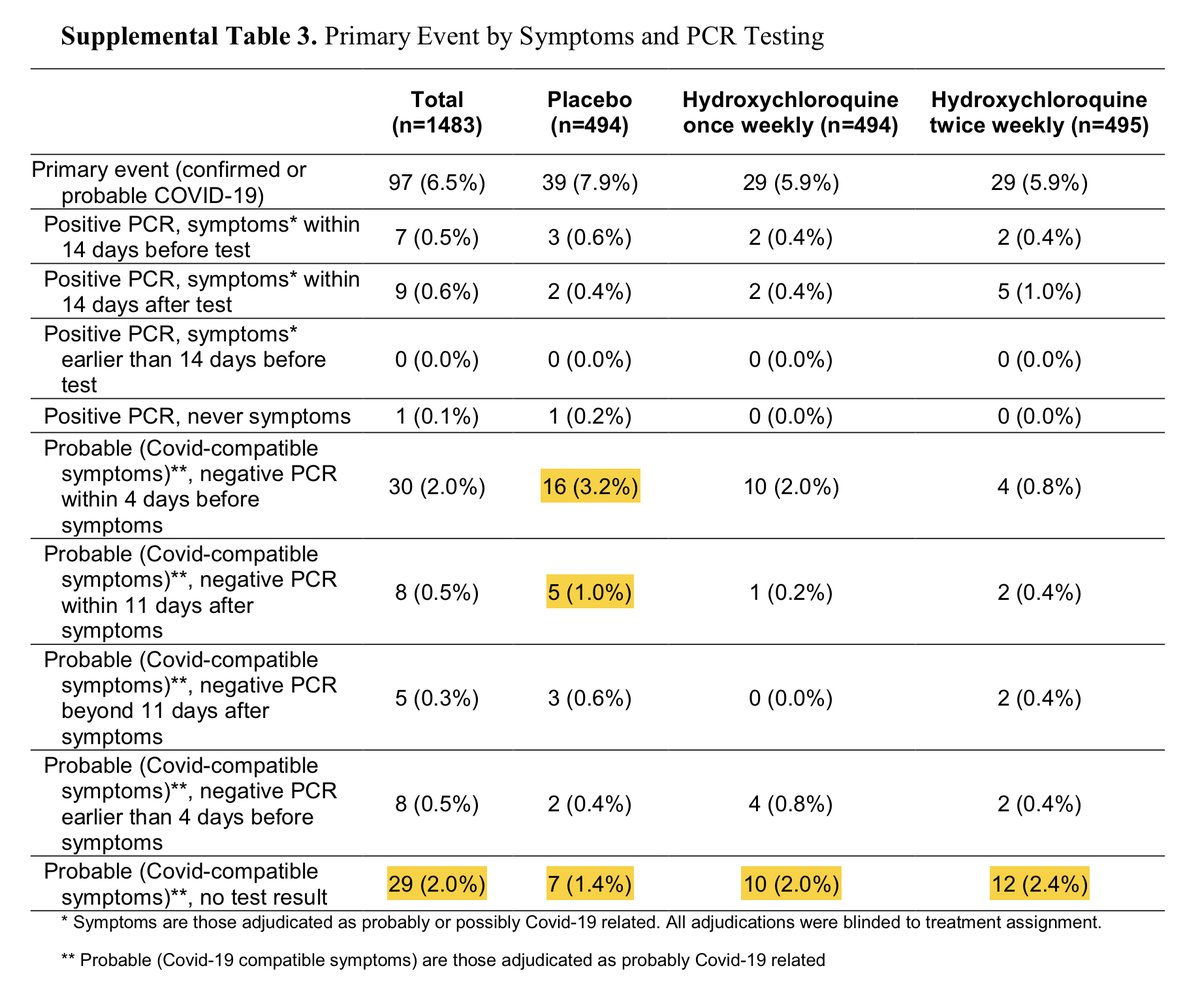
We seem to have once again lost our rigor for the PCR positivity in this meta-analysis. The entire “difference” in this study was literally driven by patients who were symptomatic and had documented PCR negativity either in the few days prior to symptoms or after symptoms
If “limiting” to PCR positivity or even just excluding those with NEGATIVE PCR’s the groups are identical.
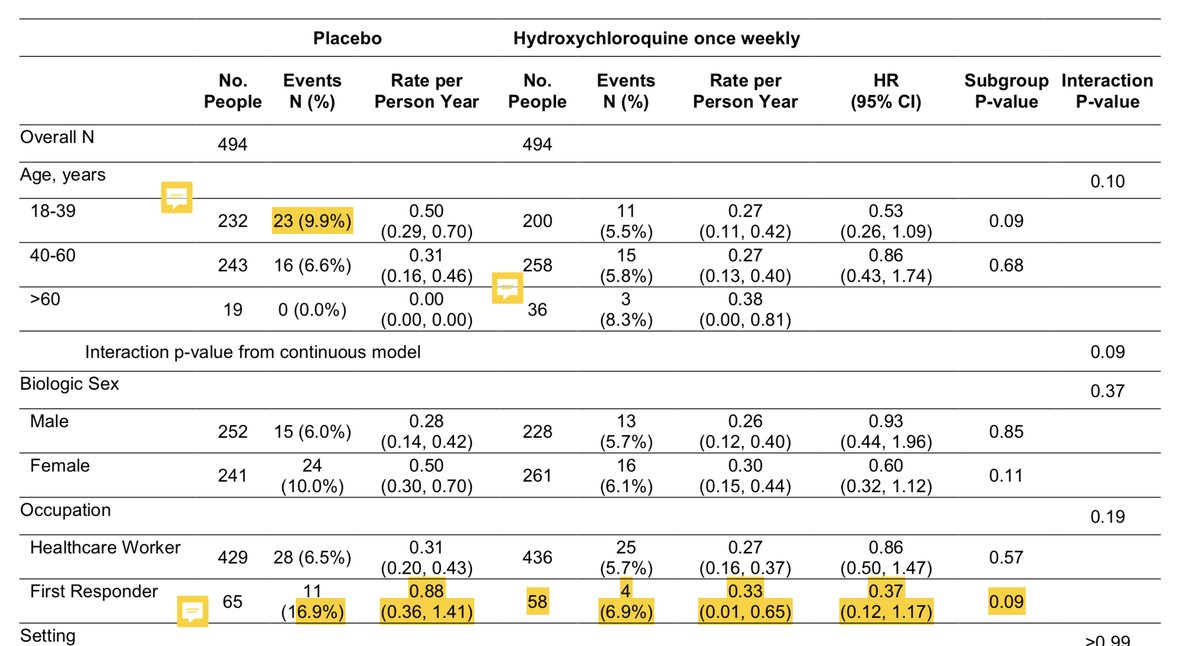
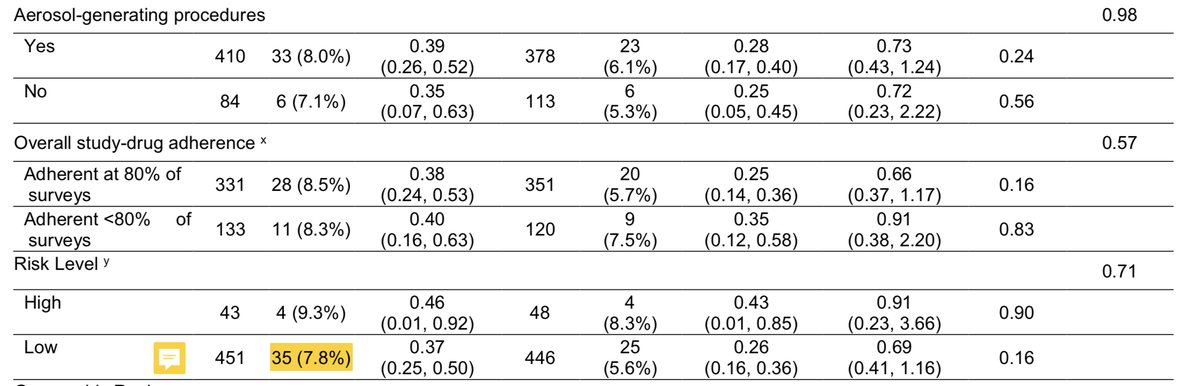
That doesn’t even get into the details of the “differences” in this trial. They are not consistent across the population (because they are not real) and the “differences” are from higher than expected rates in young pts, low-risk pts, and first-responders in placebo group
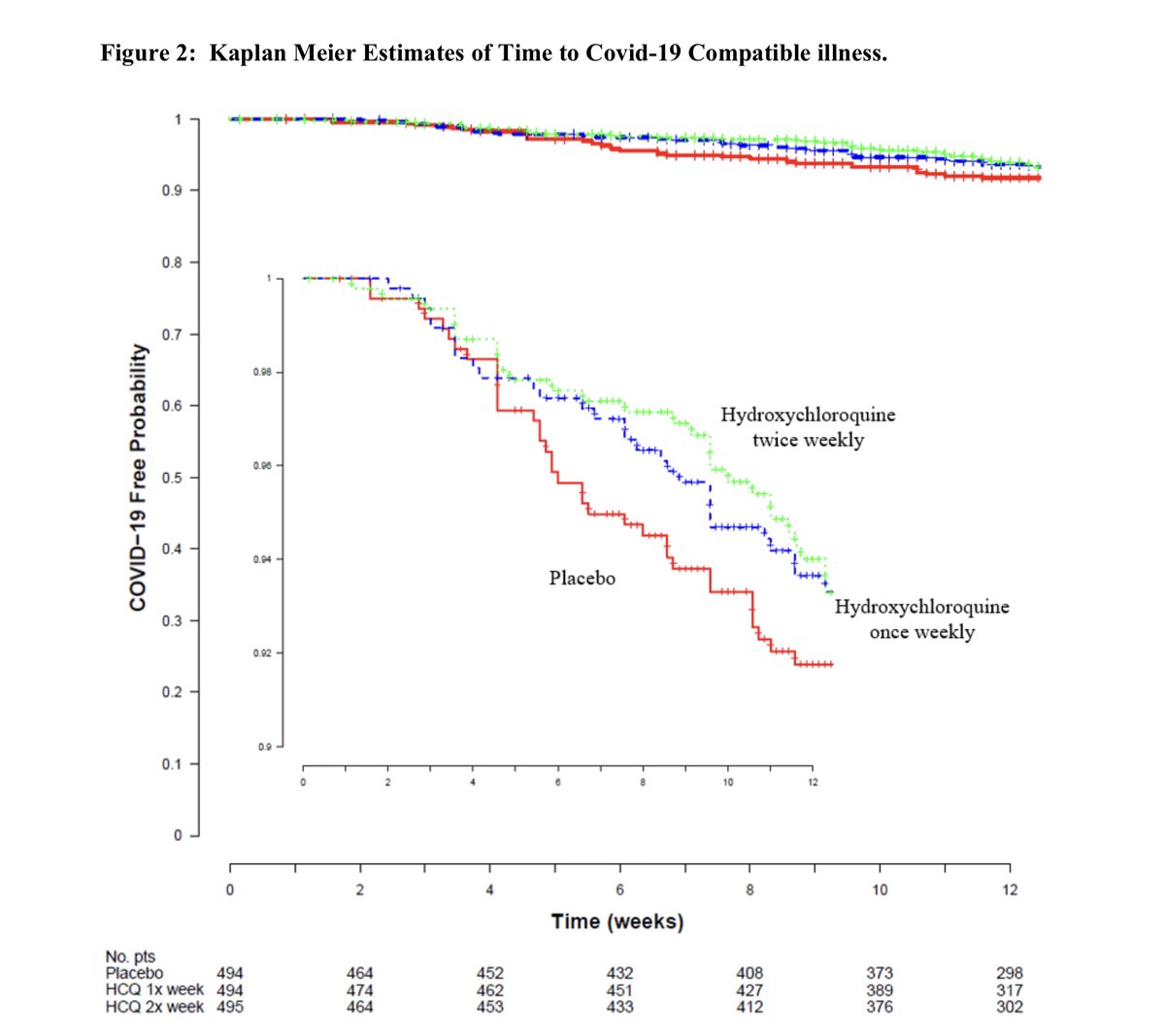
Further, the difference, if there was one, is solely from events in week 5-6. There is no plausible reason this would be the case if HCQ was truly making a difference. No difference for 4 weeks, 2% difference in event rate in weeks 5-6, no difference after.
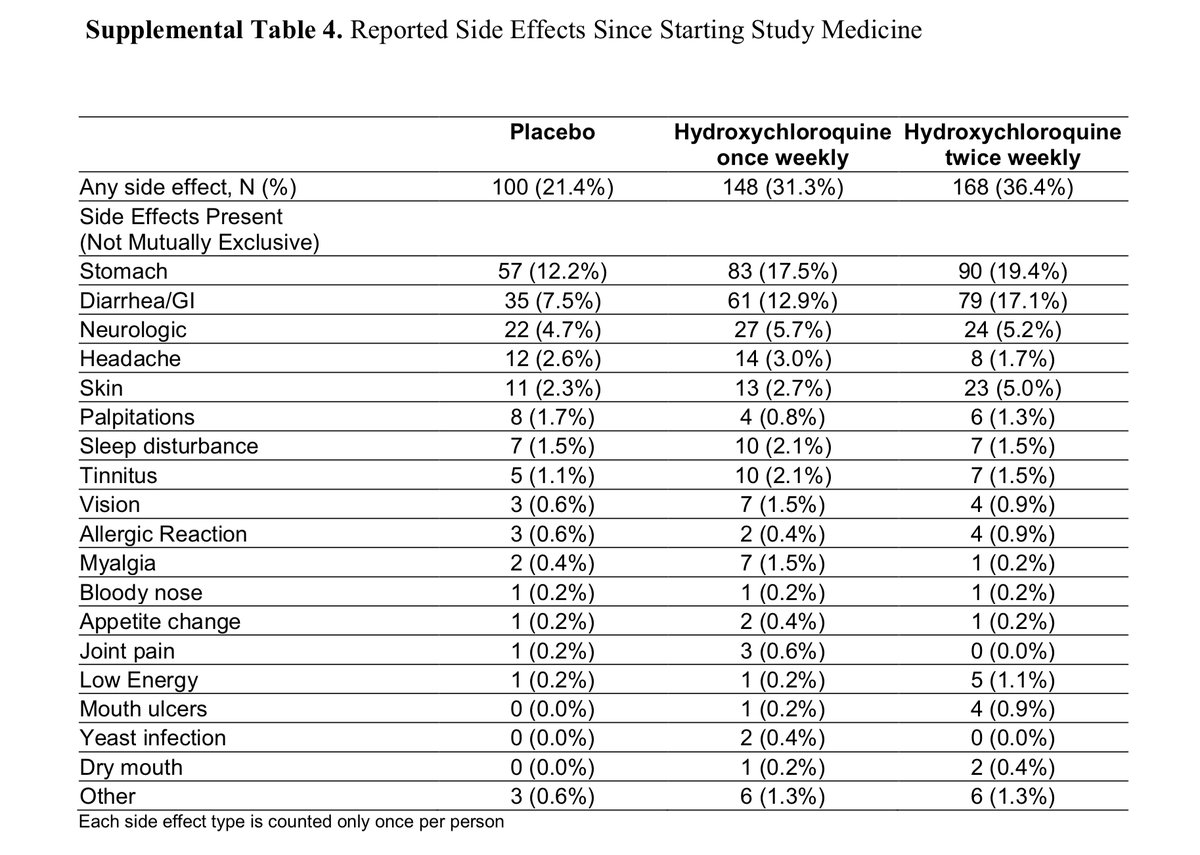
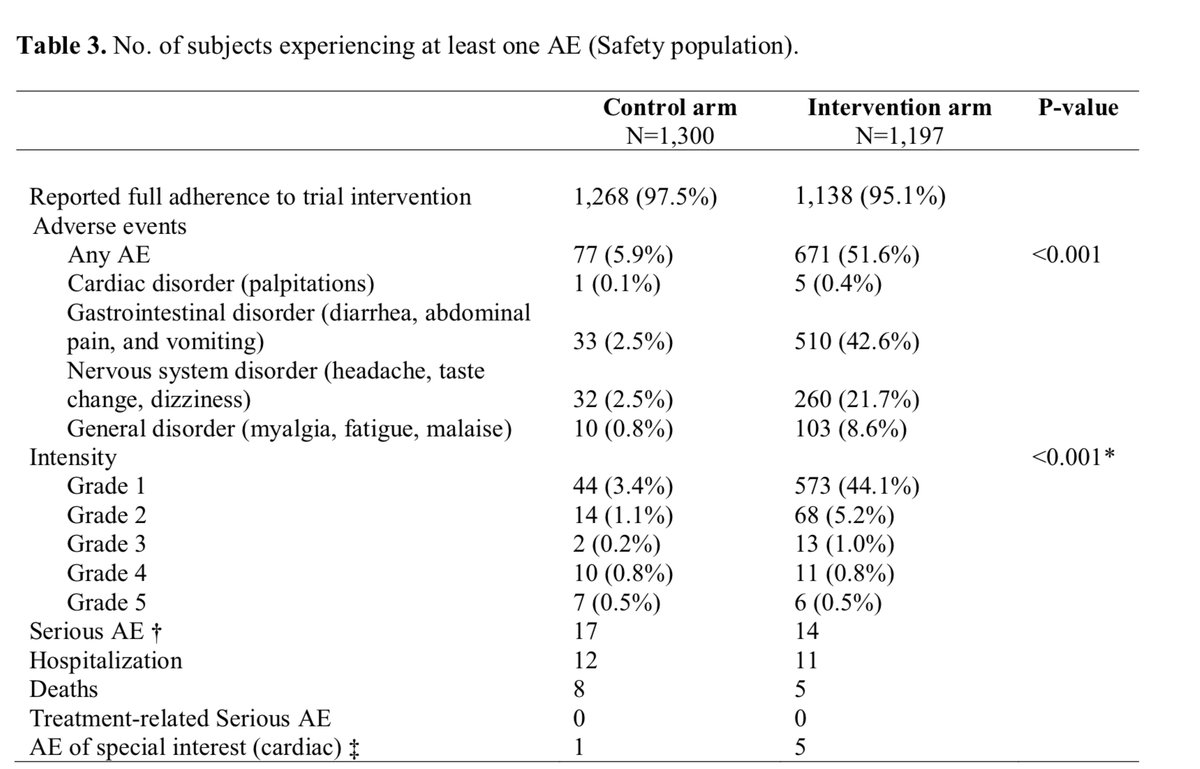
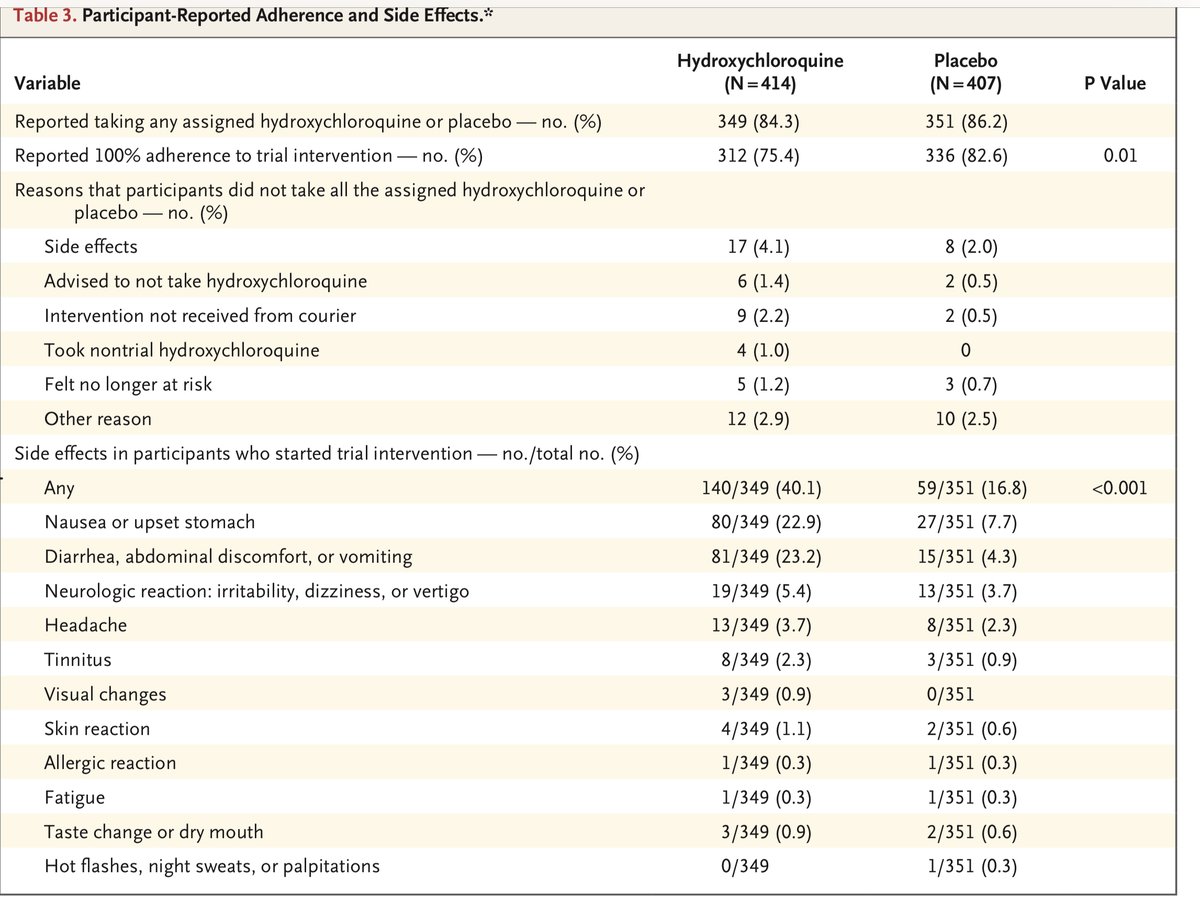
And finally, I whole heartedly disagree with the statement in the meta that “All three trials found a similar rate of adverse effects in the HCQ and no HCQ groups.” HCQ vs. placebo in the three studies: 32% vs. 21% in Rajasingham; 52% vs 6% in the Mitjà; 40% vs 17% in Boulware.
That’s a huge difference, and one adverse event is too many for a drug that a preponderance of evidence suggests doesn’t work.

 Read on Twitter
Read on Twitter