Last week, I talked about how our bodies metabolize methanol, and why that makes it so dangerous when methanol contaminates the hand sanitizers we use. Today, let’s talk about why #methanol ends up in some hand sanitizers in the first place. Let’s get started!
#handsanitizer
#handsanitizer
One way you get dangerous levels of methanol in hand sanitizer is when a manufacturer fails to distill it correctly. It’s not just hand sanitizer either. This is one of the reasons Prohibition-era moonshine had a bad rep for making people go blind.
Whether it’s booze or hand sanitizer, it makes no difference. Fermented alcohol contains ethanol and trace chemical compounds like methanol, esters, aldehydes, and acetates. Even though distillation is then used to separate the ethanol, you never get a 100% pure.
#chemtwitter
#chemtwitter
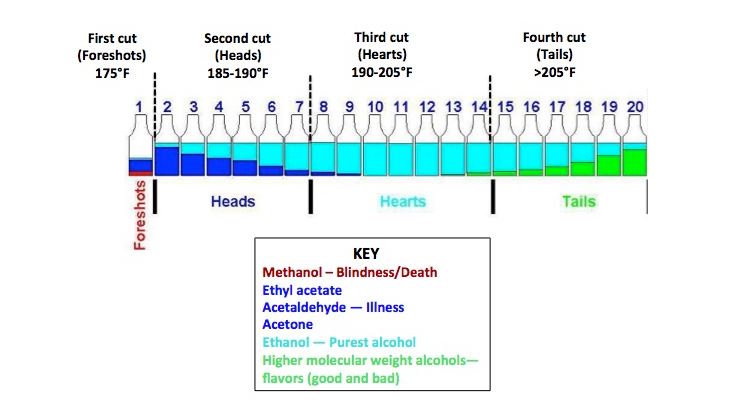
Methanol boils at a lower temperature than ethanol, and it evaporates first in distillation. Distillers must “cut” the stream of alcohol once the temperature reaches the boiling point of ethanol (171.3°F) so they can swap the collection container and toss the excess methanol.
Josh Bloom from @ACSHorg, has a brilliant post that shows the chemical compounds that can be separated out at different boiling points during distillation. https://www.acsh.org/news/2017/06/06/throw-away-first-cut-popcorn-sutton-chemistry-moonshine-11386">https://www.acsh.org/news/2017...
Once the methanol is removed, the remainder is mostly ethanol, the stuff that makes your hand sanitizer work. Even when distillation is done properly, a tiny amount of methanol will still be present, but as long as it’s below a certain threshold it’s not considered dangerous.
According to the FDA’s Temporary Policy For The Manufacturing Of Alcohol, the trace methanol present in hand sanitizer should be less than 630 ppm.
https://www.fda.gov/media/136390/download">https://www.fda.gov/media/136...
https://www.fda.gov/media/136390/download">https://www.fda.gov/media/136...
Experienced alcohol producers generally have well-trained distillers who can detect excess methanol and follow strict safety guidelines. In response to COVID, more than 1500 new distillers entered the market to boost hand sanitizer supply this year.
In an article on methanol and hand sanitizers, Chemistry World shared a statement by the FDA that said, “Almost all of the hand sanitizer manufacturers [whose products are contaminated] are newly registered facilities”. Oh, sure! Blame the new guy!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
https://www.chemistryworld.com/news/uncovering-the-sources-of-contaminated-sanitisers/4012364.article">https://www.chemistryworld.com/news/unco...
https://www.chemistryworld.com/news/uncovering-the-sources-of-contaminated-sanitisers/4012364.article">https://www.chemistryworld.com/news/unco...
The pandemic also caused a shortage of ethanol distillates, so another possible way methanol gets into hand sanitizer is the intentional/accidental purchase of high methanol distillate by manufacturers -- an action that has potentially deadly consequences.
Thanks for reading! If you have anything to share, I& #39;d love to hear  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😃" title="Lächelndes Gesicht mit geöffnetem Mund" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😃" title="Lächelndes Gesicht mit geöffnetem Mund" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund">

 Read on Twitter
Read on Twitter