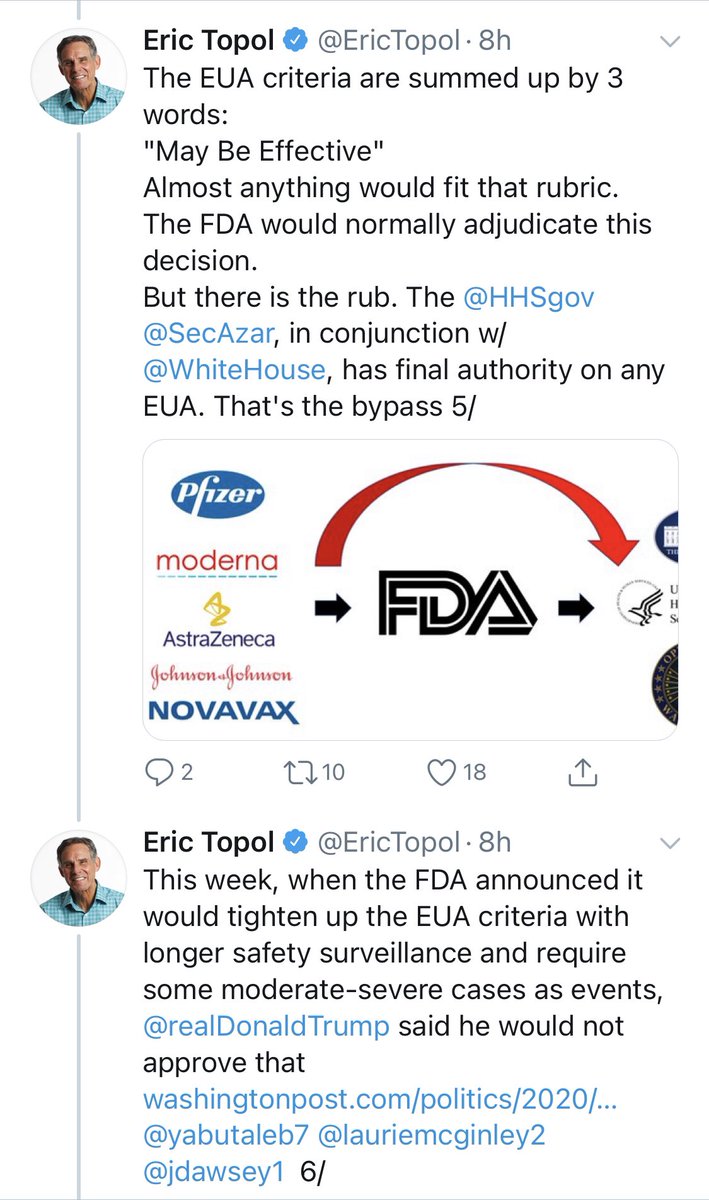
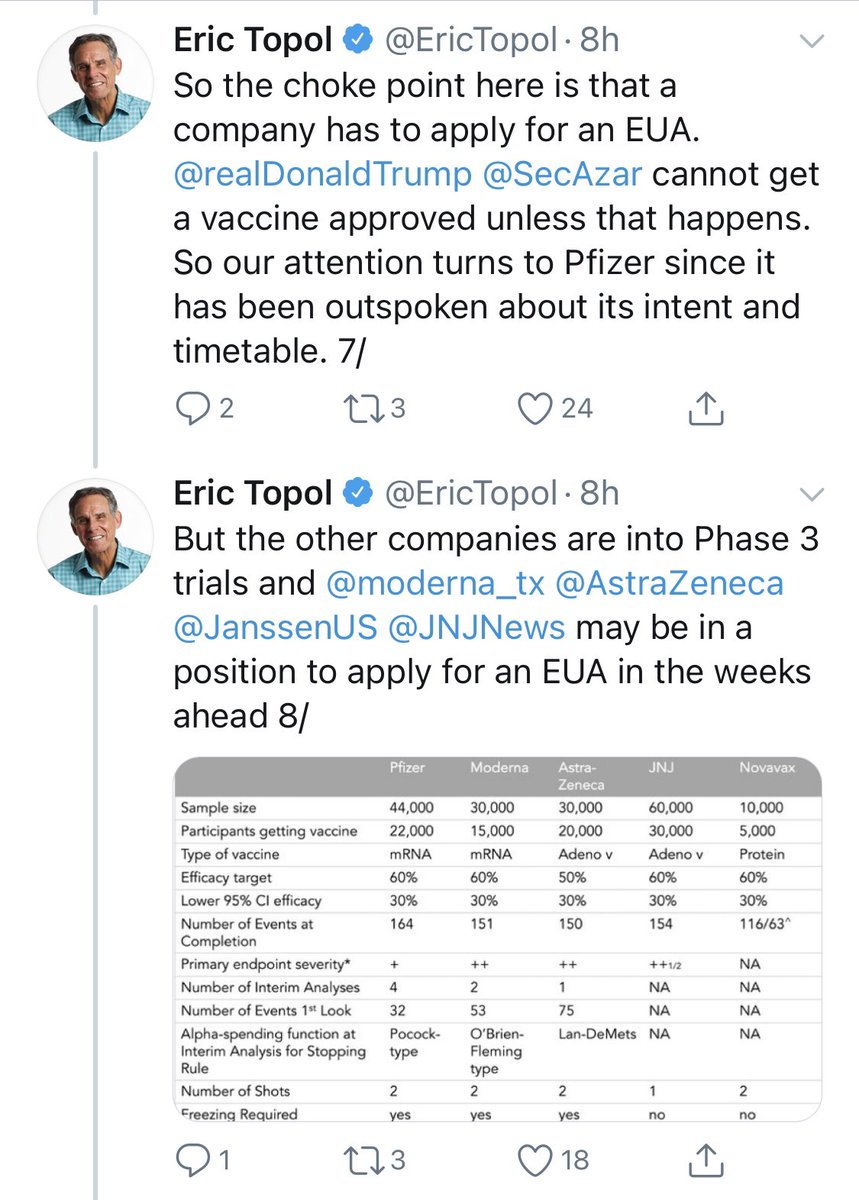
Okay. A kind soul had sent me screenshots of @EricTopol’s thread explaining his letter to Pfizer.
I have highlighted a few sections, which I will comment on.
I have highlighted a few sections, which I will comment on.
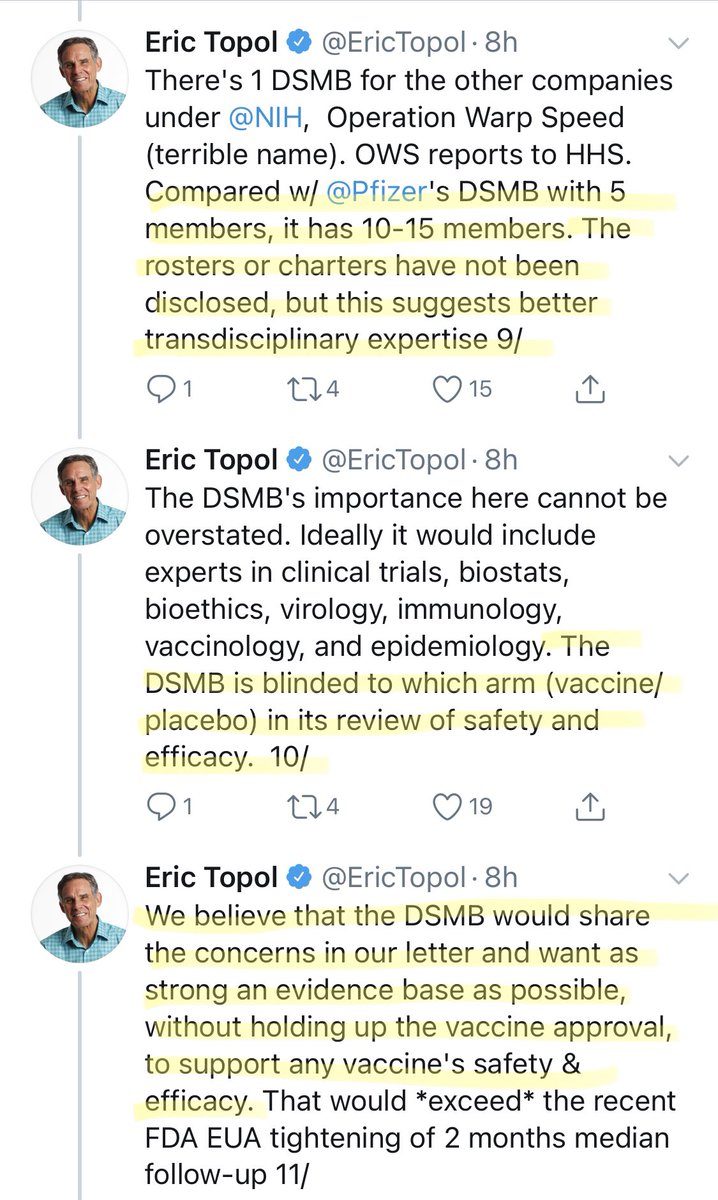
For those not familiar with the abbreviations, “DSMB” stands for “Data Safety Monitoring Board”. There are a couple of statements that deserve comment.
Here is a link to the @US_FDA guidance on DSMBs: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishment-and-operation-clinical-trial-data-monitoring-committees">https://www.fda.gov/regulator...
Here is a link to the @US_FDA guidance on DSMBs: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishment-and-operation-clinical-trial-data-monitoring-committees">https://www.fda.gov/regulator...
Here is a selection from the guidance.
Ask yourself this: In general, is a committee of 5 or a committee of 10-15 better for making decisions?
Ask yourself this: In general, is a committee of 5 or a committee of 10-15 better for making decisions?
I joke, of course. But not seeing the DSMB charters, we don’t know the composition or expertise of either the Pfizer or OWS committees.
Please note, DSMBs I have been involved with have always had allowances for “ad hoc” members with special expertise.
Please note, DSMBs I have been involved with have always had allowances for “ad hoc” members with special expertise.
Regarding the statement that the “DSMB reports to Pfizer”, it is stated in such a way as to imply a lack of independence.
DSMBs are always designed to be as independent as possible—such is needed for credibility. This structure is not unusual.
DSMBs are always designed to be as independent as possible—such is needed for credibility. This structure is not unusual.
Also note that there are multiple levels of accountability: the sponsor team (yes—they have concerns for the subjects!), IRB, regulators, and the general public.
It’s not the DSMB or bust.
It’s not the DSMB or bust.
More concern expressed on the size of the DSMB. And their temperament.
Of course the DSMB is tasked with an awesome responsibility. I doubt anyone takes that lightly.
Of course the DSMB is tasked with an awesome responsibility. I doubt anyone takes that lightly.
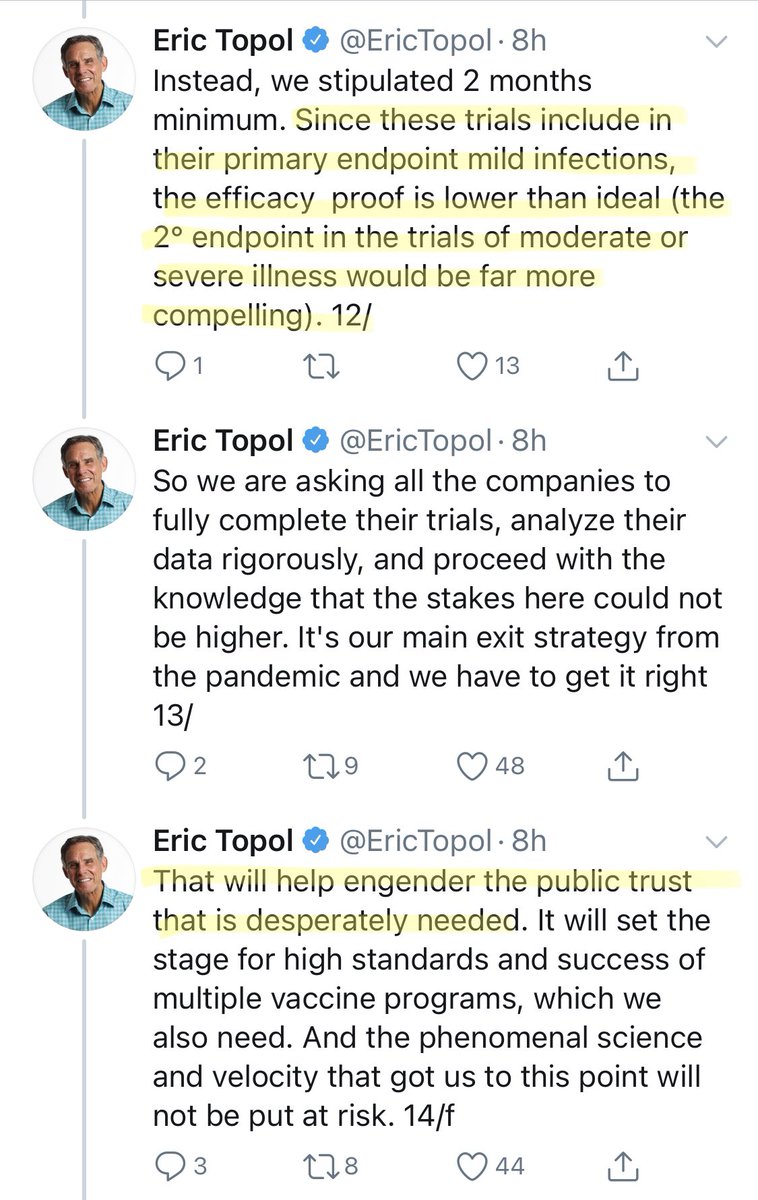
In the next tweet, there is concern expressed about the primary endpoint.
I think that this is an arguable point—why does the vaccine exist. Is it to reduce COVID severity, or to reside COVID spread?
For the former, an endpoint of death/hospitalization may be better.
I think that this is an arguable point—why does the vaccine exist. Is it to reduce COVID severity, or to reside COVID spread?
For the former, an endpoint of death/hospitalization may be better.
For the latter (preventing spread), we want to assess how the virus does or does not move from person to person—ie infection!
Both severity & spread are worth stopping. And in a perfect world one vaccine could do both.
Both severity & spread are worth stopping. And in a perfect world one vaccine could do both.
But, in choosing endpoints you need to balance speed of achieving endpoints (mild disease is more frequent than severe) with the benefit of more experienced on other elements of disease. @ProfDFrancis has recently discussed this point. https://twitter.com/profdfrancis/status/1309126333021249537">https://twitter.com/profdfran...
One point I may have gotten out of order: does the DSMB have access to only blinded data?
No way to know without seeing the charter, but in DSMBs I have been involved with, they had access to *unblinded* data.
This is a discussion WAY to big for a tweet, though.
No way to know without seeing the charter, but in DSMBs I have been involved with, they had access to *unblinded* data.
This is a discussion WAY to big for a tweet, though.
So, how to we engender public trust?
The way I would do it is by avoiding fear-mongering, sticking to what is known, and not turning on the hype machine to 11.
The way I would do it is by avoiding fear-mongering, sticking to what is known, and not turning on the hype machine to 11.

 Read on Twitter
Read on Twitter