Ok...Honeymoon is over & now time to dissect the exciting new data presented @ #ESMO2020 for #GEC #KN590 #CM649 #ATTRCN4 #CM577
1st, thank you to all the pts who participated!
& 2nd, congrats to all the investigators involved!
It& #39;s fantastic to have +ve studies! Lets dive deep:
1st, thank you to all the pts who participated!
& 2nd, congrats to all the investigators involved!
It& #39;s fantastic to have +ve studies! Lets dive deep:
*Caution
I think the facts are accurate - please correct if not. (seeking the truth here)
My opinions are my opinions.
~15 min read (it& #39;s complicated)
No CME offered unfortunately https://abs.twimg.com/emoji/v2/... draggable="false" alt="😒" title="Unerfreutes Gesicht" aria-label="Emoji: Unerfreutes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😒" title="Unerfreutes Gesicht" aria-label="Emoji: Unerfreutes Gesicht"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">
Enjoy...
I think the facts are accurate - please correct if not. (seeking the truth here)
My opinions are my opinions.
~15 min read (it& #39;s complicated)
No CME offered unfortunately
Enjoy...
Background:
IO monotx effective in a subgroup of GEC pts in 1L+:
1. MSI-H
2. High PDL1 (cut-off at least CPS 10 22C3)
3. low tumor burden
4. PS0
5. Asian > Western pts
6. SCC > AC
7. GC > EGJ
Outside of above, most pts are better-served w chemo based on crossing #yinyang curves
IO monotx effective in a subgroup of GEC pts in 1L+:
1. MSI-H
2. High PDL1 (cut-off at least CPS 10 22C3)
3. low tumor burden
4. PS0
5. Asian > Western pts
6. SCC > AC
7. GC > EGJ
Outside of above, most pts are better-served w chemo based on crossing #yinyang curves
Some studies that provided this background:
#GEA
KN12 ATTRCN2 KN59
CM32 KN61 KN62
JAVLN300&100
#EsoSCCAC
KN28 KN180 KN181
#EsoSCC
ATTRCN3, ONO-4538-07
#AST
MSI-H (bunch of KN study subsets pooled)
#yinyang:
#GEA
KN12 ATTRCN2 KN59
CM32 KN61 KN62
JAVLN300&100
#EsoSCCAC
KN28 KN180 KN181
#EsoSCC
ATTRCN3, ONO-4538-07
#AST
MSI-H (bunch of KN study subsets pooled)
#yinyang:
These led to
1) nivo 3L+ approval #GEA (Asia)
2) pembro 3L+ approval PDL1 CPS>1 22C3 #GEA (US)
3) pembro MSI-H 2L+ any solid tumor #GEC
4) pembro 2L+ CPS>10 22C3 #EsoSCC
5) nivo 2L+ approval #EsoSCC
These are the approvals as of & #39;the glorious day& #39; (TGD) 9/21/20 @ #ESMO2020
1) nivo 3L+ approval #GEA (Asia)
2) pembro 3L+ approval PDL1 CPS>1 22C3 #GEA (US)
3) pembro MSI-H 2L+ any solid tumor #GEC
4) pembro 2L+ CPS>10 22C3 #EsoSCC
5) nivo 2L+ approval #EsoSCC
These are the approvals as of & #39;the glorious day& #39; (TGD) 9/21/20 @ #ESMO2020
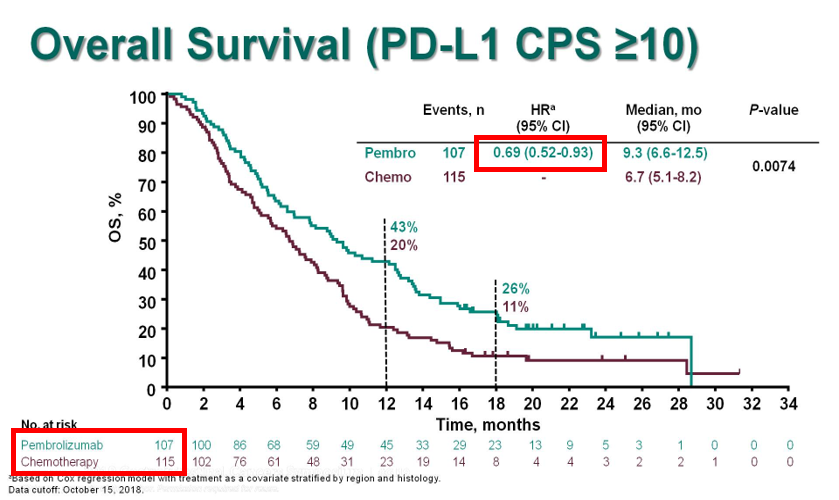
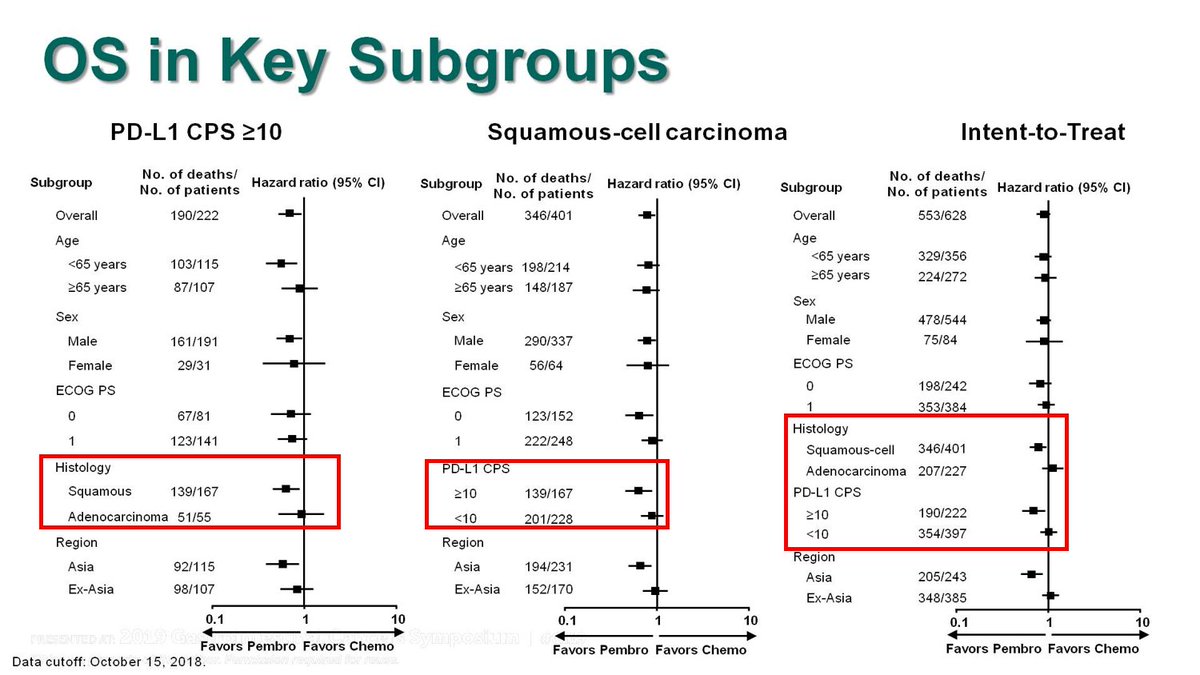
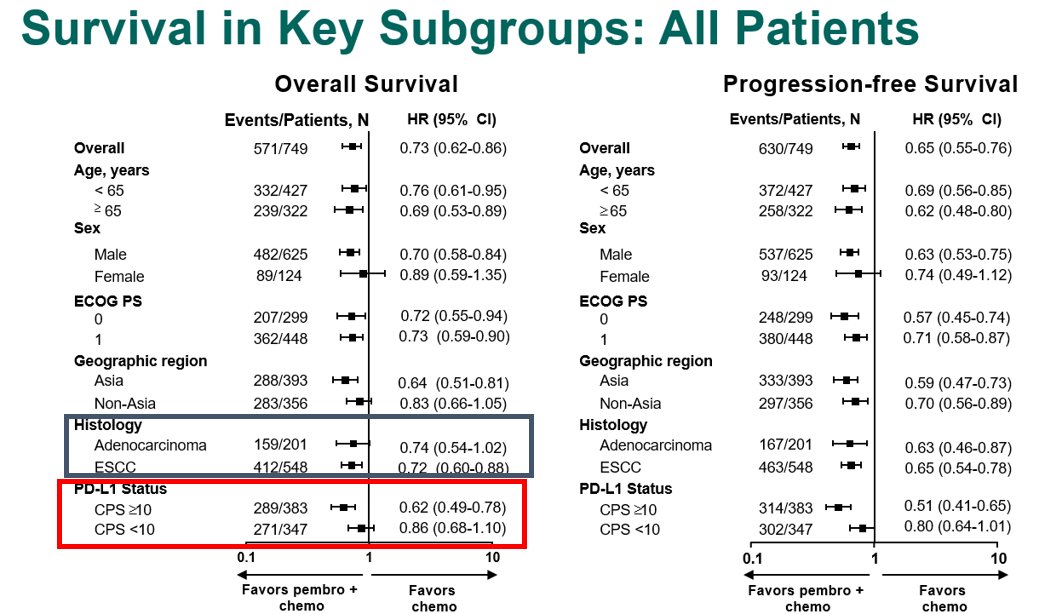
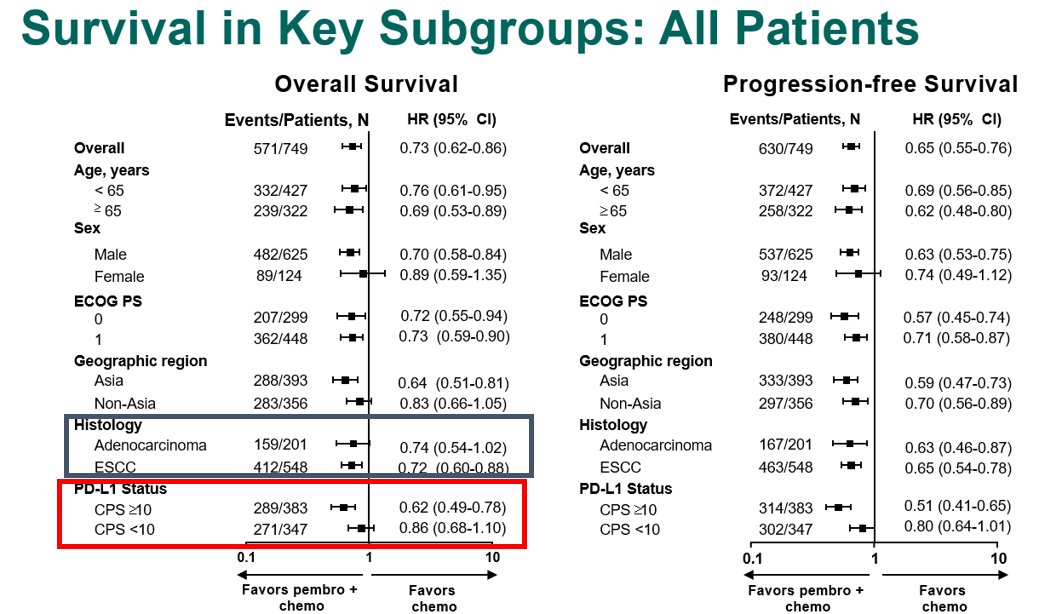
For the KN181 study, although the ITT, the all SCC (including <CPS10) & the all CPS>10 (including AC) demonstrated improved OS when lumped with SCC>10, Forrest plot clearly shows lack of benefit for AC, and PDL1<1. FDA approved only #SCC CPS >10. See #Cresendo plots later@ #KN62.
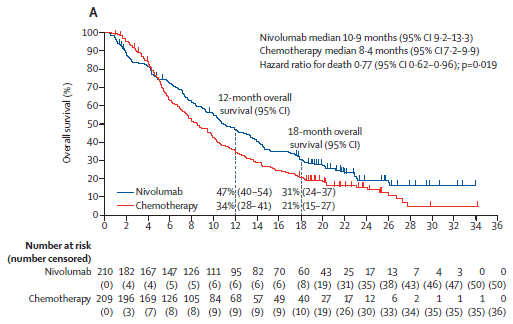
ATTRCN3 #SCC 2L study - akin to KN181
1) mini #yinyang curve
2) there were 18 non-Asian pts. Check out KN181 Forrest plot https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian
3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:
1) mini #yinyang curve
2) there were 18 non-Asian pts. Check out KN181 Forrest plot
3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:
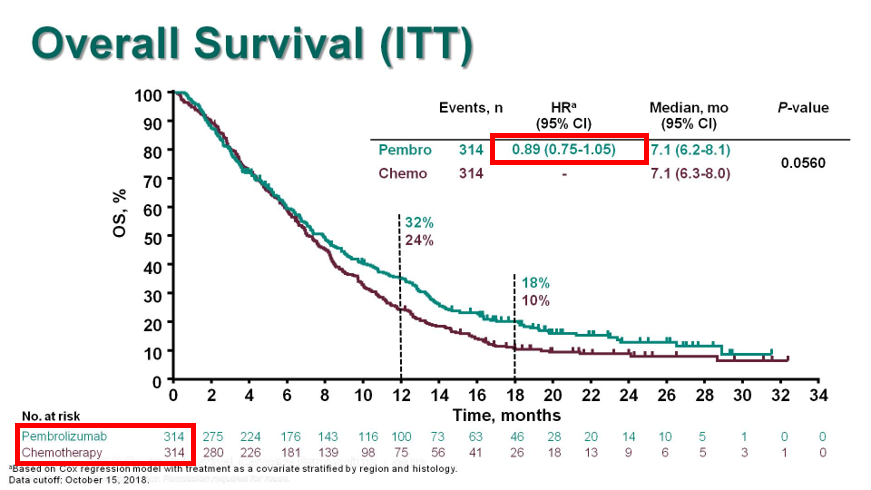
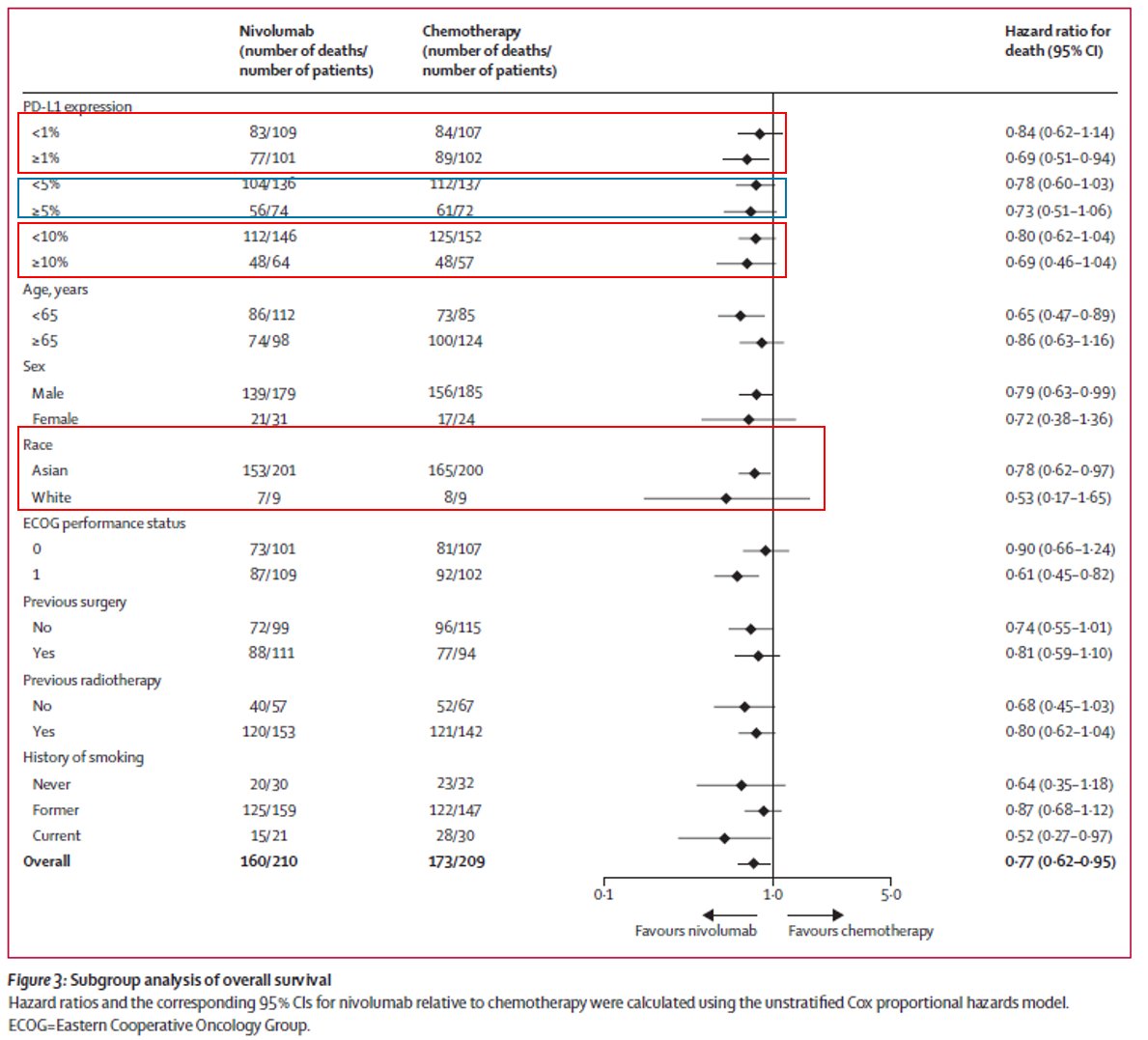
Specifically for 1L, no approvals to date. The only phase 3 1L study before TGD: KN062 N=750 1:1:1. Pertinents:
1. 3-arm R placbo contrlld study CPS >1 22C3 only
open labl pembro monotx
& cis/FP+pembro/placbo
2. monotx arm - #yinyang even CPS>10 N~100 per arm #donoharm plz!
1. 3-arm R placbo contrlld study CPS >1 22C3 only
open labl pembro monotx
& cis/FP+pembro/placbo
2. monotx arm - #yinyang even CPS>10 N~100 per arm #donoharm plz!
3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)
4. Chemo arms: #Crescendo https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit by
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit by https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)
5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD.
Remember only CPS>1 22C3 pts!
4. Chemo arms: #Crescendo
5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD.
Remember only CPS>1 22C3 pts!
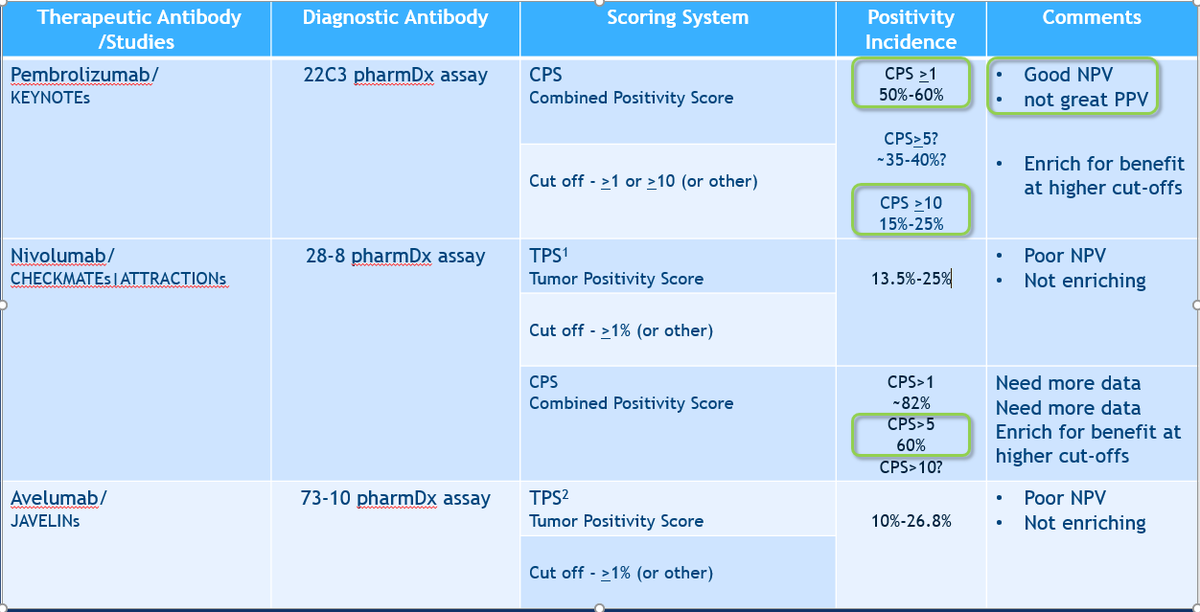
Last background- Biomarker assays to date:
pembro 22C3 CPS>1 ~60%, >10 ~15-25%
nivo 28-8 TPS>1 ~15-30%
Avelu 73-10 TPS>1 ~10-26.8%
Notice, nivo not previously been evaluated by CPS
Diff diagnostic Abs=diff +ve rate =diff predictive performance
Which will you do?
pembro 22C3 CPS>1 ~60%, >10 ~15-25%
nivo 28-8 TPS>1 ~15-30%
Avelu 73-10 TPS>1 ~10-26.8%
Notice, nivo not previously been evaluated by CPS
Diff diagnostic Abs=diff +ve rate =diff predictive performance
Which will you do?
Enter TGD:
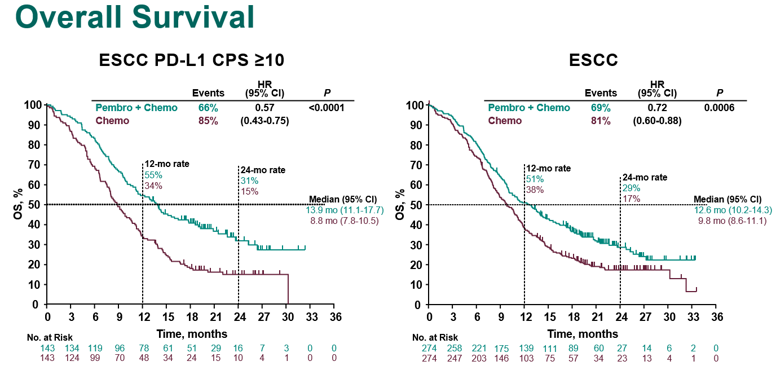
Let& #39;s start with #KN590 #EsoSCCAC
N=749 pts 1:1 Cis/FP +pembro/placebo
CPS>10 ~50% & Asian 50%, SCC 73% (!) of pts
Results great!
But VERY similar to KN181 ( https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.
Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
Let& #39;s start with #KN590 #EsoSCCAC
N=749 pts 1:1 Cis/FP +pembro/placebo
CPS>10 ~50% & Asian 50%, SCC 73% (!) of pts
Results great!
But VERY similar to KN181 (
Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC?
So #KN590, even though the ITT & all #SCC appear to benefit, those #crescendo plots get less pronounced, due to many pts added that don& #39;t benefit, compared to the #SCC CPS >10 & all CPS >10 plots.
HRs of 0.57 & 0.62 went to 0.72 & 0.73 - what must the isolated HR AC/CPS<10 be?
HRs of 0.57 & 0.62 went to 0.72 & 0.73 - what must the isolated HR AC/CPS<10 be?
Q: What is the % of Adeno in #KN590 that are CPS>10? This is important when comparing to other studies, and is not reported in the Forrest plot
Q: Will the FDA see past this #KN590 illusion, as it did with KN181, or no?  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
#KN590 My Thoughts:
Practice change for 1L CPS >10 (for sure #EsoSCC, but probably also #EsoAC)
I need to see #EsoSCC CPS <10 & <1 isolated to really comment if it is yay or nay. Hopefully yay, but https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤷♂️" title="Achselzuckender Mann" aria-label="Emoji: Achselzuckender Mann">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤷♂️" title="Achselzuckender Mann" aria-label="Emoji: Achselzuckender Mann">
#KN590 My Thoughts:
Practice change for 1L CPS >10 (for sure #EsoSCC, but probably also #EsoAC)
I need to see #EsoSCC CPS <10 & <1 isolated to really comment if it is yay or nay. Hopefully yay, but
P.S. EsoAC = GEJAC = Cardia in my book.
P.P.S. Can we stop doing studies lumping SCC&AC so tweetorials don& #39;t have to be so long to explain?
P.P.P.S. For now ok to lump GC&EGJAC #GEA
P.P.S. Can we stop doing studies lumping SCC&AC so tweetorials don& #39;t have to be so long to explain?
P.P.P.S. For now ok to lump GC&EGJAC #GEA
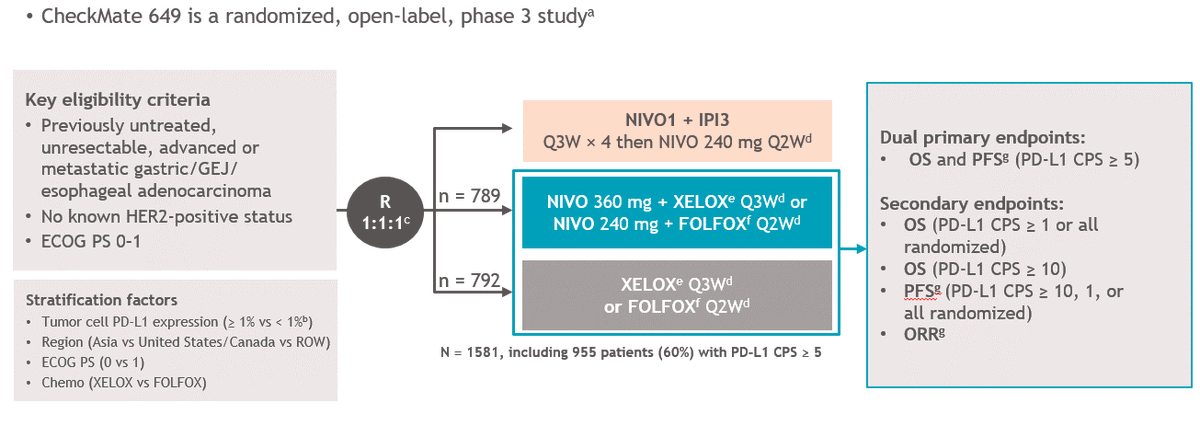
Next up, #CM649
N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))
FOLFOX/CapeOx nivo/ipi
**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)
N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))
FOLFOX/CapeOx nivo/ipi
**Nivo/Ipi terminated early - no public info o/w
Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, &
Amending trial structure & primary endpoints mid-trial - yikes! FOLFOX-nivo arm was not in the original design!
**Nivo/Ipi #CM649 will likely be a #YinYang plot in all-comers, the original endpoint of TPS >1% & likely even the CPS >5. Will be interesting to see this soon plz.
**Nivo/Ipi #CM649 will likely be a #YinYang plot in all-comers, the original endpoint of TPS >1% & likely even the CPS >5. Will be interesting to see this soon plz.
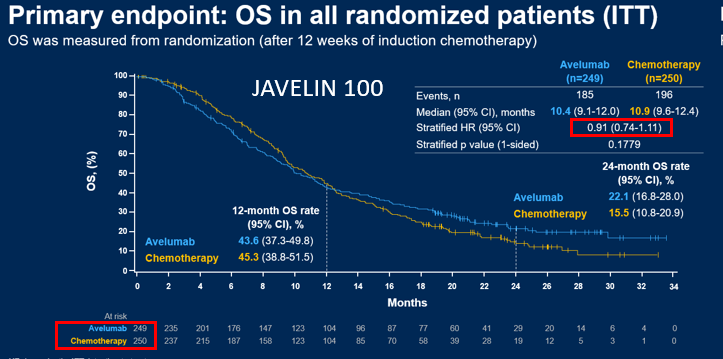
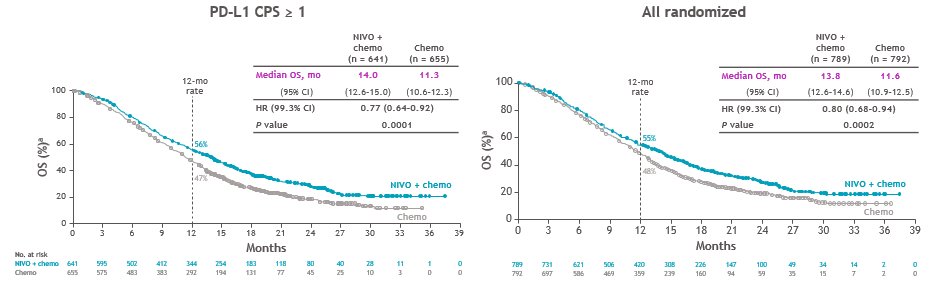
Biomarker contrasts of chemo arms:
CM649 N=1581 any PDL1 28-8 Ab
CPS>1 n=1296 (82%)
CPS>5 n= 955 (60%)
KN062 N=500 only CPS>1 22C3Ab
CPS>1 N=500 Usually ~60% of all pts tested are +ve
CPS>10 n=200 (~20%) (~40% of the CPS>1 pts)
CPS>5 (~35%ish)
Summary: 28-8 calls >pts +ve.
CM649 N=1581 any PDL1 28-8 Ab
CPS>1 n=1296 (82%)
CPS>5 n= 955 (60%)
KN062 N=500 only CPS>1 22C3Ab
CPS>1 N=500 Usually ~60% of all pts tested are +ve
CPS>10 n=200 (~20%) (~40% of the CPS>1 pts)
CPS>5 (~35%ish)
Summary: 28-8 calls >pts +ve.
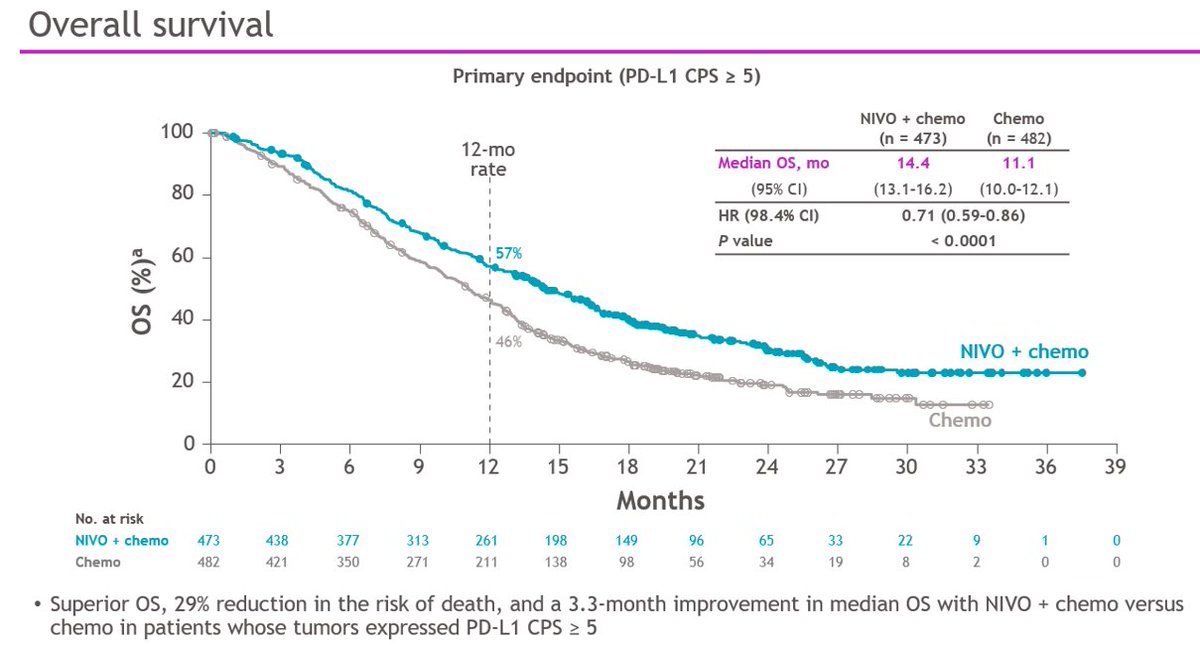
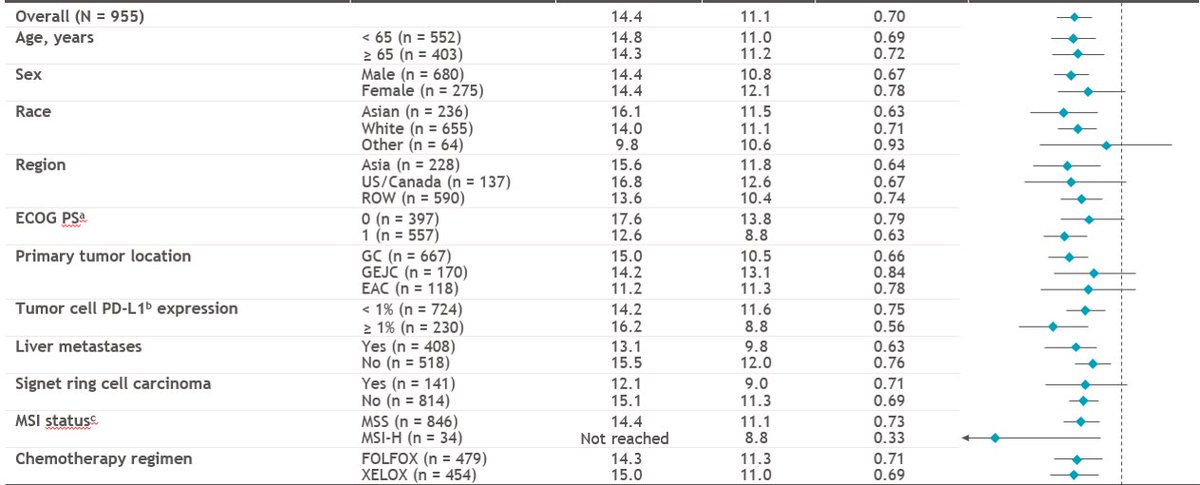
So...nice result for CPS >5 28-8, the amended primary endpt. Why different than Chemo comparison of #KN062 for both CPS>1 & CPS>10 22C3?? (and subject of the pre-test poll) See later  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
#Crescendo
#Crescendo
1. CPS>1? Let& #39;s add ~150 pts to the nivo arm (+ the 473 CPS>5)
2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)
dampened #crescendo plots
What do the CPS <1 and CPS <5 and CPS 1-4 look like? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not?
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)
dampened #crescendo plots
What do the CPS <1 and CPS <5 and CPS 1-4 look like?
PS @pashtoonkasi MSI-H incidence exactly where it should be for #CM649: 3-4% in the metatastic setting. #KN062 is inflated because >90% of MSI-H tumors are CPS>1, while MSS are only ~50-60% CPS >1, so nearly double the expected rate there.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👀" title="Augen" aria-label="Emoji: Augen"> the MSI-H HR with nivo? 0.33! #CM649
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👀" title="Augen" aria-label="Emoji: Augen"> the MSI-H HR with nivo? 0.33! #CM649
Sidestep to #ATTRCN04
N=724 1:1 All Asian, All comers, any PDL1
SOX/CapeOx +nivo/placebo
PFS endpt met, BUT OS endpt not met (mos ~17m! HR0.9)
Why neg compared to #CM649? More cross-over, more later line therapies in Asia? Maybe But also: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
N=724 1:1 All Asian, All comers, any PDL1
SOX/CapeOx +nivo/placebo
PFS endpt met, BUT OS endpt not met (mos ~17m! HR0.9)
Why neg compared to #CM649? More cross-over, more later line therapies in Asia? Maybe But also:
To the pre-test:
Q: What are the differences b/w #KN062 #CM649 & #ATTRCN4 that led to only #CM649 positive for OS?
1. Oxaliplatin #CM649 & #ATTRCN4 vs Cisplatin #KN062?
2. Double/Triple sample size to have more power?
3. Open label vs Blinded
4. Amended primary endpoint mid-trial
Q: What are the differences b/w #KN062 #CM649 & #ATTRCN4 that led to only #CM649 positive for OS?
1. Oxaliplatin #CM649 & #ATTRCN4 vs Cisplatin #KN062?
2. Double/Triple sample size to have more power?
3. Open label vs Blinded
4. Amended primary endpoint mid-trial
1. Oxali vs Cis. Least likely of all.
Oxali better synergy? but Cis was +ve in #KN590 (and other tumor types+IO). ATTRCN4 had oxali but neg OS.
2/4. https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">er sample size & CPS>5 more
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">er sample size & CPS>5 more https://abs.twimg.com/emoji/v2/... draggable="false" alt="👊" title="Fisted hand" aria-label="Emoji: Fisted hand">?Yep
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👊" title="Fisted hand" aria-label="Emoji: Fisted hand">?Yep
3. https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐘" title="Elefant" aria-label="Emoji: Elefant">in the Zoom: CM649 the only open label trial. Do we need to Rehash need for placebos?
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐘" title="Elefant" aria-label="Emoji: Elefant">in the Zoom: CM649 the only open label trial. Do we need to Rehash need for placebos? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤷♂️" title="Achselzuckender Mann" aria-label="Emoji: Achselzuckender Mann">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤷♂️" title="Achselzuckender Mann" aria-label="Emoji: Achselzuckender Mann">
Oxali better synergy? but Cis was +ve in #KN590 (and other tumor types+IO). ATTRCN4 had oxali but neg OS.
2/4.
3.
Q: What will the FDA do with the #CM649 study? Approve only by the amended primary endpt CPS>5? Or also >1, or all comers? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">
#CM646 My Thoughts:
Obvious approval of CPS>5. (MSI-H included in here)
I will not treat my pts with this until I see the isolated results CPS<1& CPS1-4
#CM646 My Thoughts:
Obvious approval of CPS>5. (MSI-H included in here)
I will not treat my pts with this until I see the isolated results CPS<1& CPS1-4
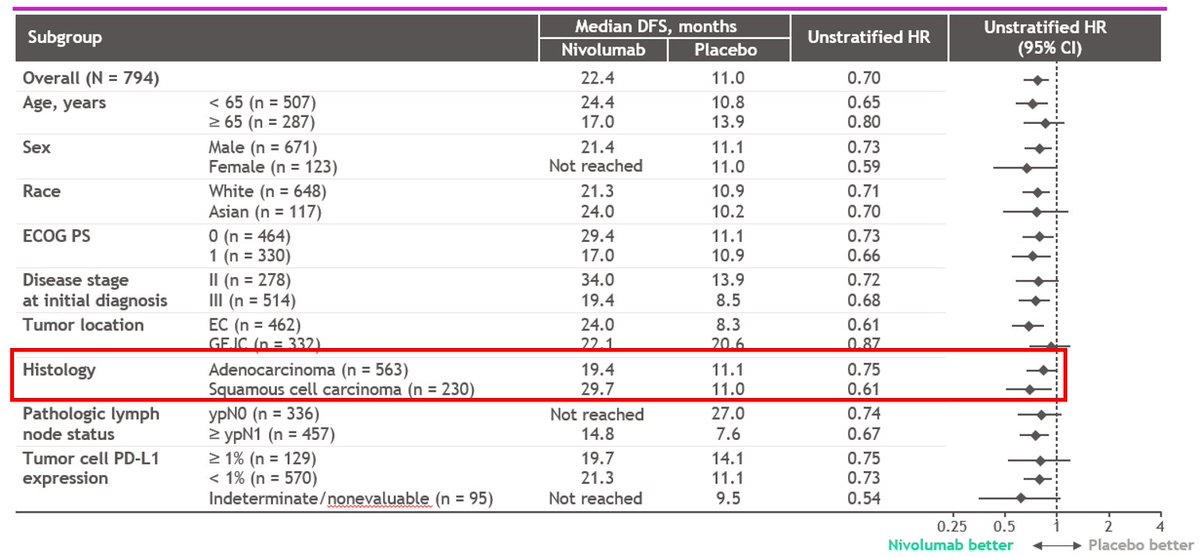
#CM577
N=794
R 2:1 nivo vs placebo in high risk ypT+N+ #EsoSCCAC
70% AC, 30% SCC
Primary endpt DFS
Fantastic result - I agree w @DrRonanKelly, 10% absolute DFS here will likely translate to OS benefit.
The sobering part: ~40% recur by 1yr, ~50% by 2yr, ~60% by 3yr. Plateau!(?)
N=794
R 2:1 nivo vs placebo in high risk ypT+N+ #EsoSCCAC
70% AC, 30% SCC
Primary endpt DFS
Fantastic result - I agree w @DrRonanKelly, 10% absolute DFS here will likely translate to OS benefit.
The sobering part: ~40% recur by 1yr, ~50% by 2yr, ~60% by 3yr. Plateau!(?)
As expected Forest plot shows the benefit from SCC >> AC.
Q: What do the DFS curves look like for AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">(Probably a more pronounced tuning fork)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">(Probably a more pronounced tuning fork)
Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)
Let& #39;s see soon?
Q: What do the DFS curves look like for AC?
Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5?
Let& #39;s see soon?
I will leave the Triplet chemo vs CRT for another day, but #KN585 periop doublet chemo +pembro/placebo results will be interesting here for the Adeno comparison with #CM577 adeno component
So will the FDA approve adjuvant nivo by these DFS data alone (the primary endpoint), or require OS as well (secondary endpoint)?
The results are exciting & it& #39;s great to have better outcomes for pts w #GEC
I make the points above b/c it is our responsibility to identify who is & isn& #39;t deriving benefit from IO, so we get it to those who do, but with intentions to figure out why it isn& #39;t working in others.
I make the points above b/c it is our responsibility to identify who is & isn& #39;t deriving benefit from IO, so we get it to those who do, but with intentions to figure out why it isn& #39;t working in others.
"Targeted therapies for targeted populations."
Immune checkpoint inhibitors are targeted therapies, and they are not immune to this concept.
Cheers
Immune checkpoint inhibitors are targeted therapies, and they are not immune to this concept.
Cheers

 Read on Twitter
Read on Twitter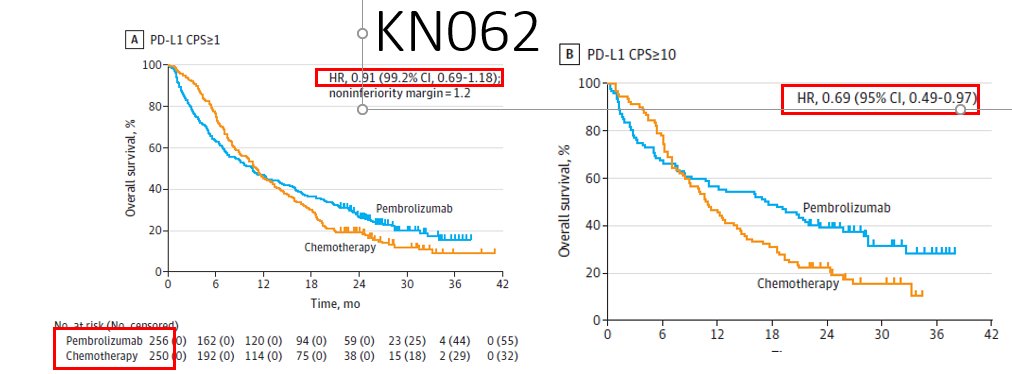



 re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:" title="ATTRCN3 #SCC 2L study - akin to KN1811) mini #yinyang curve2) there were 18 non-Asian pts. Check out KN181 Forrest plot https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:">
re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:" title="ATTRCN3 #SCC 2L study - akin to KN1811) mini #yinyang curve2) there were 18 non-Asian pts. Check out KN181 Forrest plot https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:">
 re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:" title="ATTRCN3 #SCC 2L study - akin to KN1811) mini #yinyang curve2) there were 18 non-Asian pts. Check out KN181 Forrest plot https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:">
re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:" title="ATTRCN3 #SCC 2L study - akin to KN1811) mini #yinyang curve2) there were 18 non-Asian pts. Check out KN181 Forrest plot https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> re Asian vs ex-Asian3) TPS 28-8 PDL1 & #39;did not predict outcome& #39; but trend to more benefit with higher level:">
 curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
 curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
 curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
 curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!" title="3. Chemo arms: -ve for superiority (HR0.85, p=0.05 #alphasplittinggalore)4. Chemo arms: #Crescendohttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🎼" title="Partitur" aria-label="Emoji: Partitur">curves suggesting magnitude of benefit byhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">ing gradient (higher PDL1 level?)5. This was the 1st/only Phase3 data of chemo+IO in 1L #GEC before TGD. Remember only CPS>1 22C3 pts!">
 ). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
 ). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
 ). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="Enter TGD:Let& #39;s start with #KN590 #EsoSCCACN=749 pts 1:1 Cis/FP +pembro/placeboCPS>10 ~50% & Asian 50%, SCC 73% (!) of ptsResults great!But VERY similar to KN181 (https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">). More #crescendo plots.Q: The CPS >10 curve is shown, what& #39;s the CPS <10 look like by SCC and AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
 Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
 Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
 Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)" title="Next up, #CM649N=originally 1266 1:1 (633 each) all comers (Primary endpt >1% TPS (28-8 Ab))FOLFOX/CapeOx nivo/ipi**Nivo/Ipi terminated early - no public info o/w https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">Major Amendments @ dif times: Added 3rd arm FOLFOX-nivo, CPS>5, & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">& #39;d size of chemo arms: to ~1600 (~800 each)">
 #Crescendo" title="So...nice result for CPS >5 28-8, the amended primary endpt. Why different than Chemo comparison of #KN062 for both CPS>1 & CPS>10 22C3?? (and subject of the pre-test poll) See later https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> #Crescendo" class="img-responsive" style="max-width:100%;"/>
#Crescendo" title="So...nice result for CPS >5 28-8, the amended primary endpt. Why different than Chemo comparison of #KN062 for both CPS>1 & CPS>10 22C3?? (and subject of the pre-test poll) See later https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> #Crescendo" class="img-responsive" style="max-width:100%;"/>
 & Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="1. CPS>1? Let& #39;s add ~150 pts to the nivo arm (+ the 473 CPS>5) 2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)dampened #crescendo plotsWhat do the CPS <1 and CPS <5 and CPS 1-4 look like? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="1. CPS>1? Let& #39;s add ~150 pts to the nivo arm (+ the 473 CPS>5) 2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)dampened #crescendo plotsWhat do the CPS <1 and CPS <5 and CPS 1-4 look like? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
 & Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="1. CPS>1? Let& #39;s add ~150 pts to the nivo arm (+ the 473 CPS>5) 2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)dampened #crescendo plotsWhat do the CPS <1 and CPS <5 and CPS 1-4 look like? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">
& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">" title="1. CPS>1? Let& #39;s add ~150 pts to the nivo arm (+ the 473 CPS>5) 2. All comers? Let& #39;s add ~150pts more (+ the 150pts CPS1-4 and 473 CPS>5)dampened #crescendo plotsWhat do the CPS <1 and CPS <5 and CPS 1-4 look like? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">& Forest plot did not include these subgroups - why not?https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">">


 (Probably a more pronounced tuning fork)Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)Let& #39;s see soon?" title="As expected Forest plot shows the benefit from SCC >> AC. Q: What do the DFS curves look like for AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">(Probably a more pronounced tuning fork)Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)Let& #39;s see soon?" class="img-responsive" style="max-width:100%;"/>
(Probably a more pronounced tuning fork)Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)Let& #39;s see soon?" title="As expected Forest plot shows the benefit from SCC >> AC. Q: What do the DFS curves look like for AC? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht">(Probably a more pronounced tuning fork)Q: What do the DFS curves look like for AC CPS <1 vs 1-4 vs >5? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Denkendes Gesicht" aria-label="Emoji: Denkendes Gesicht"> (maybe even more pronounced tuning forks for CPS<5)Let& #39;s see soon?" class="img-responsive" style="max-width:100%;"/>


