Celebrating our new publication in @atvbahajournals! https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎉" title="Partyknaller" aria-label="Emoji: Partyknaller"> We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiers
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎉" title="Partyknaller" aria-label="Emoji: Partyknaller"> We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiers https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n
https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.315261">https://www.ahajournals.org/doi/10.11...
https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.315261">https://www.ahajournals.org/doi/10.11...
Why should you care about this work? TL;DR, inflammation is key for controlling aortic valve stenosis (AVS) progression, and we think engineered models of AVS disease can bring us one step closer to finding ways to control inflammation and slow or stop AVS. 2/n
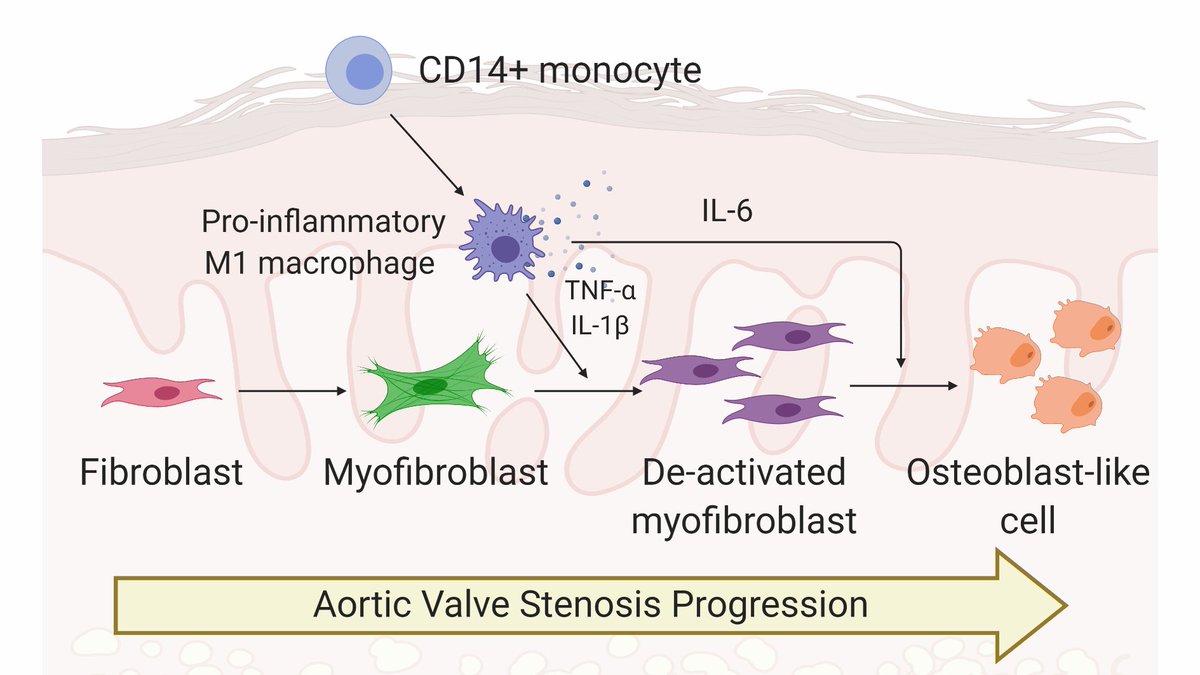
AVS causes stiffening of valve tissue. With AVS, hearts work harder to pump blood through the valve (think rusty hinge), causing heart failure. Valves typically undergo fibrotic thickening before becoming bone-like and calcified. Thanks to @BioRender for great valve cartoons! 3/n
AVS is currently treated using surgical valve replacements. We& #39;d like to learn more about the biological processes that regulate valve stiffening to develop effective non-surgical treatments using drugs to treat AVS patients. 4/n
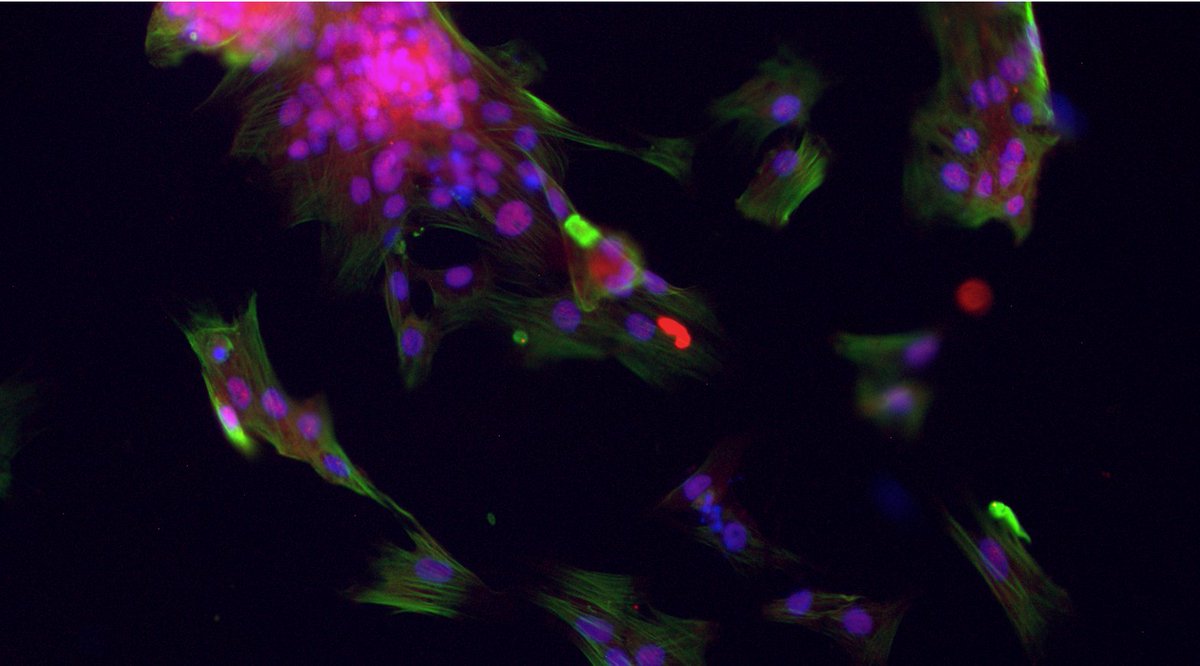
Valvular interstitial cells, or VICs, are the primary drivers of AVS progression. VICs (purple) activate to cells known as myofibroblasts (green), which cause aberrant collagen deposition and tissue stiffening. 5/n
Over time, VICs/myofibroblasts can become osteoblast-like cells (red), which cause calcification of valve tissue, making the tissue bone-like. Little is known about the biological processes that control the switch from myofibroblasts to osteoblast-like cells. 6/n
Like in virtually every other disease, inflammation is a hallmark of AVS progression. We https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">hypothesized
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">hypothesized https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> factors secreted from pro-inflammatory macrophages would regulate VIC myofibroblast activation and promote their differentiation toward osteoblast-like cells. 7/n
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> factors secreted from pro-inflammatory macrophages would regulate VIC myofibroblast activation and promote their differentiation toward osteoblast-like cells. 7/n
To test our hypothesis, we used hydrogel biomaterials as culture platforms to mimic the stiffness of the valve tissue microenvironment. We treated VICs cultured on these gels with macrophage conditioned media to test the effects of secreted factors on VIC phenotype. 8/n
Interestingly, VICs treated with conditioned media from inflammatory macrophages completely deactivated! But WHY? We think inflammatory factors (specifically TNFa and IL-1b) drive VIC myofibroblast deactivation. 9/n
VICs treated with inflammatory factors are also proliferative (cells with green nuclei), which we think is important for valve leaflet thickening. 10/n
We also found inflammatory factors (specifically IL-6) drive osteogenic activation of VICs. Together, we think inflammatory factors deactivate myofibroblasts and promote osteoblast-like differentiation. 11/n
This project was a follow-up to our @ScienceTM study, showing inflammation after TAVR may regulate myofibroblast deactivation. Next steps will be to correlate inflammatory factors with TAVR patient outcomes. Check out our work here: https://stm.sciencemag.org/content/11/509/eaav3233.abstract">https://stm.sciencemag.org/content/1...
12/n
12/n
Even though this year has been relentlessly difficult, @JoeCGrim and I had a great time working on this project together. We hope you enjoy reading our paper (or at least, enjoyed this thread!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">) Stay healthy, everyone! 13/13
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">) Stay healthy, everyone! 13/13

 Read on Twitter
Read on Twitter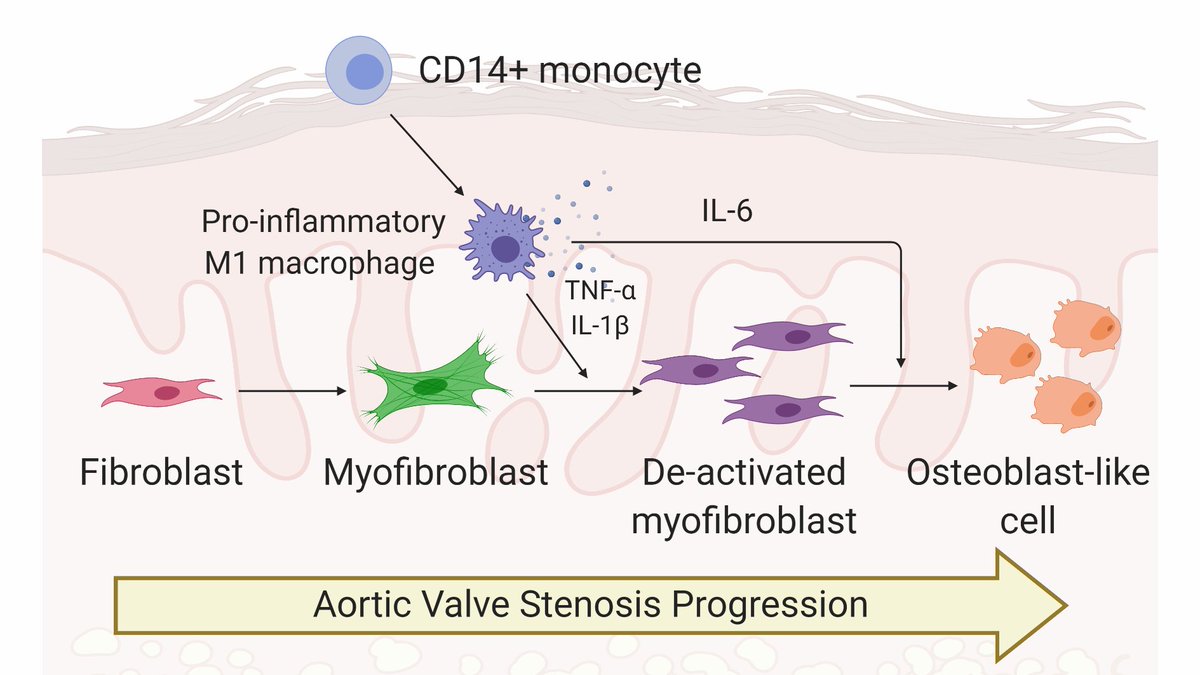 We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n https://www.ahajournals.org/doi/10.11..." title="Celebrating our new publication in @atvbahajournals!https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎉" title="Partyknaller" aria-label="Emoji: Partyknaller"> We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n https://www.ahajournals.org/doi/10.11..." class="img-responsive" style="max-width:100%;"/>
We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n https://www.ahajournals.org/doi/10.11..." title="Celebrating our new publication in @atvbahajournals!https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎉" title="Partyknaller" aria-label="Emoji: Partyknaller"> We show inflammatory macrophages drive an intermediate valve cell phenotype that may promote the switch from fibrosis to calcification in valve tissue. Awesome working w @JoeCGrim! @BioFrontiershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">1/n https://www.ahajournals.org/doi/10.11..." class="img-responsive" style="max-width:100%;"/>





 ) Stay healthy, everyone! 13/13" title="Even though this year has been relentlessly difficult, @JoeCGrim and I had a great time working on this project together. We hope you enjoy reading our paper (or at least, enjoyed this thread! https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">) Stay healthy, everyone! 13/13" class="img-responsive" style="max-width:100%;"/>
) Stay healthy, everyone! 13/13" title="Even though this year has been relentlessly difficult, @JoeCGrim and I had a great time working on this project together. We hope you enjoy reading our paper (or at least, enjoyed this thread! https://abs.twimg.com/emoji/v2/... draggable="false" alt="😉" title="Zwinkerndes Gesicht" aria-label="Emoji: Zwinkerndes Gesicht">) Stay healthy, everyone! 13/13" class="img-responsive" style="max-width:100%;"/>


