1/ A nurse at a company central to Trump& #39;s COVID plasma therapy push said workers were in danger. @CSLPlasma gave unsafe masks, she alleged, as it recruited once-infected donors w/ cash bonuses.
This reporting journey ends w/ a document somebody forged & unanswered questions …
This reporting journey ends w/ a document somebody forged & unanswered questions …
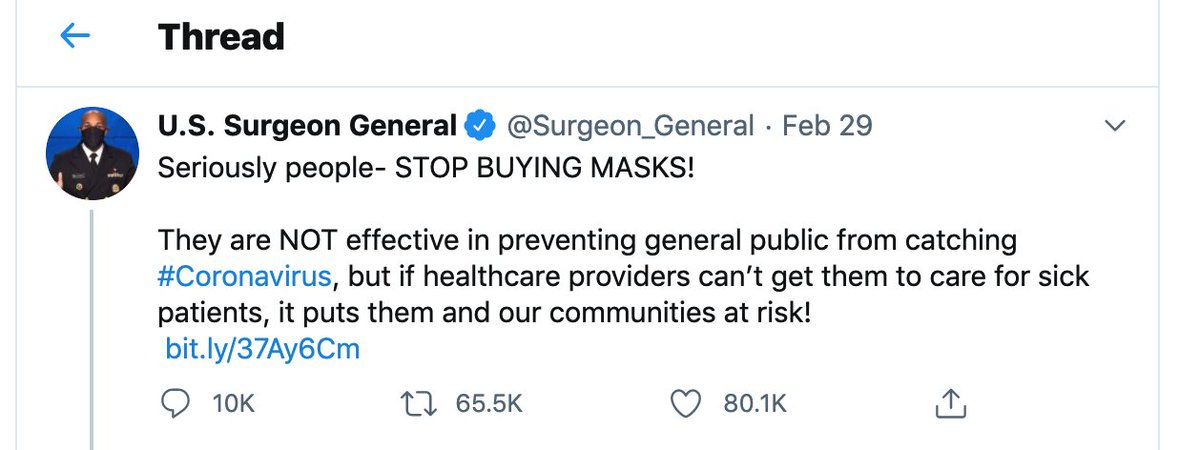
2/ @HSteadman_RN said workers& #39; fears began in March, when CSL still wasn’t supplying and mandating masks. In fairness this was around the same time this famous Tweet made the rounds…
3/ In April, Steadman learned @CSLPlasma wanted her and colleagues to examine & work w/ donors who had survived the virus. Their plasma contains antibodies that health officials hope can be turned into medicine to help severely ill patients recover.
4/ There were - and are - lots of questions about immunity, reinfection and when folks are no longer contagious. Steadman told her bosses she had no choice but to go on leave because she feared for her safety. Here’s part of the notice she gave in April.
5/ She also filed a complaint with @OSHA_DOL, hoping to force her employer to supply masks that can filter out particles containing the virus.
(CSL repeatedly told me the company follows OSHA, CDC and local health guidelines)
(CSL repeatedly told me the company follows OSHA, CDC and local health guidelines)
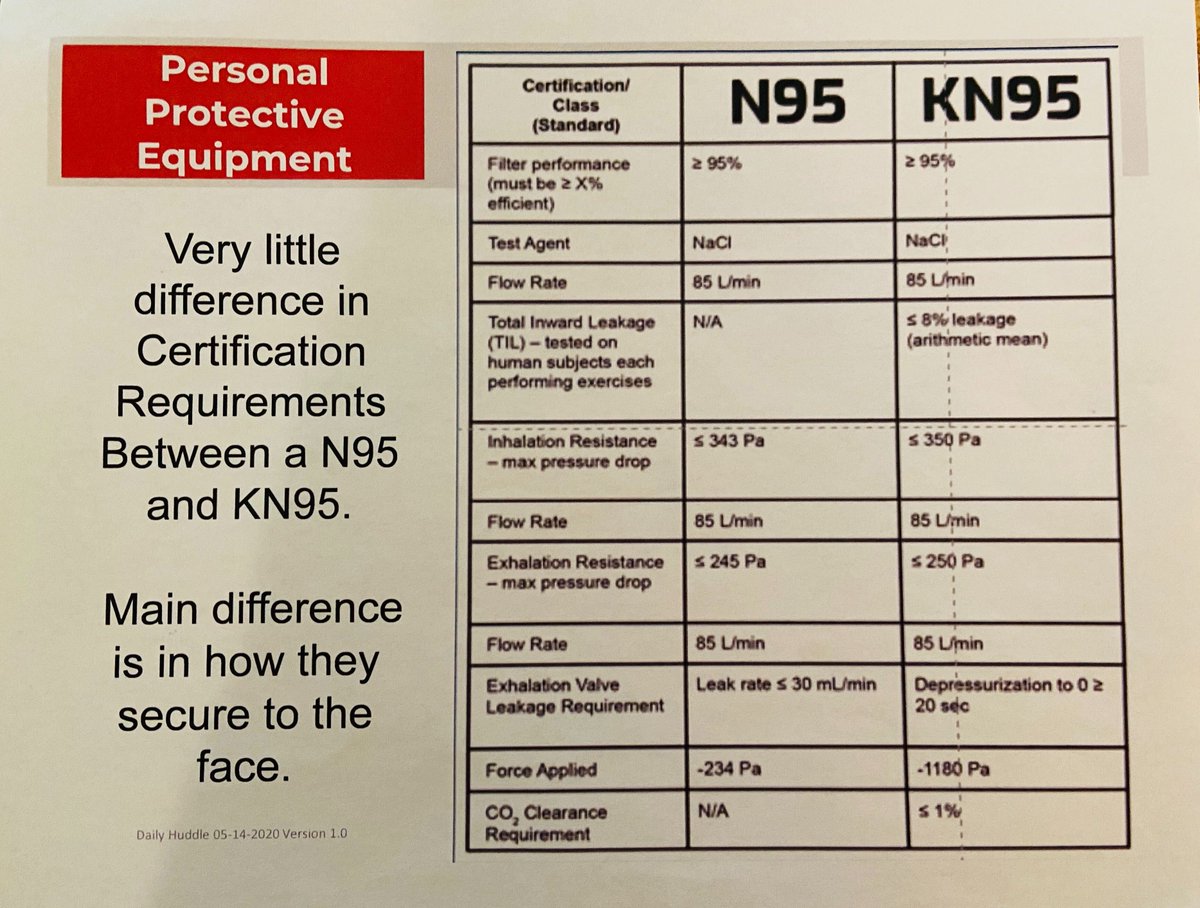
6/ She returned in June, after CSL distributed masks it said were just as good as the elusive N95 respirator, which filters out 95% of particles that could carry the virus. They were the Chinese KN95s ...
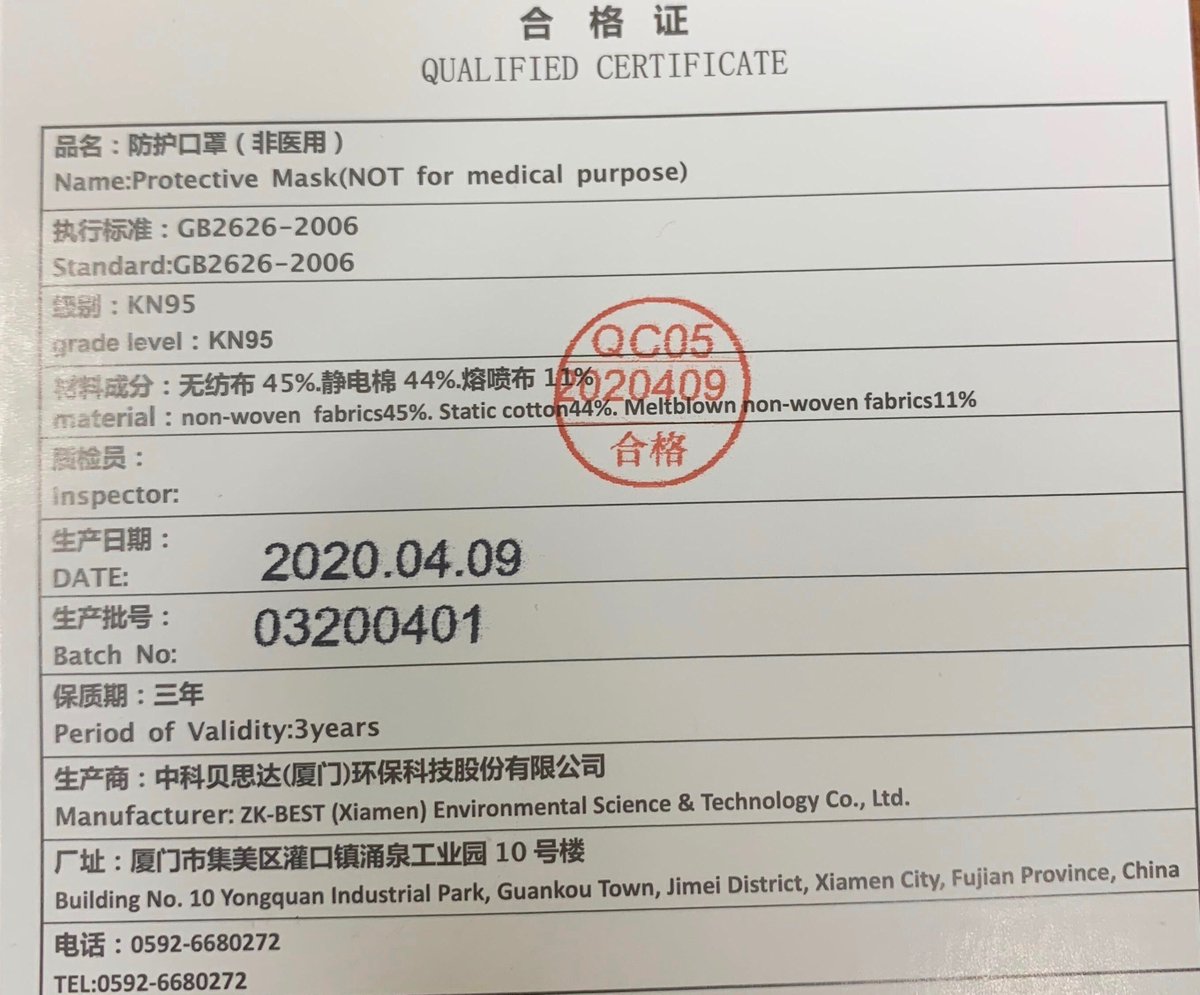
7/ Back at the donation center in McKeesport, PA, she was handed a mask just before entering a tiny closed door room to examine a donor. “There were just red flags all over it,” she said. Here it is …
8/ She plunged her hand into a blue box of 50 or so masks. She pulled out a sheet of paper, hoping for proof of authenticity. Instead, she read aloud the first line of English on the note: “NOT for medical purpose.”
9/ Again, she told CSL managers she would not work until the company provided safer masks. She called OSHA and was told to file a new complaint, so she did …
10/ After months of waiting for @OSHA_DOL to act, Steadman grew frustrated and reached out to ProPublica for help.
“Aren& #39;t they the people who are supposed to protect me?” she told me. “And now we’re in the middle of a pandemic and where are they?””
“Aren& #39;t they the people who are supposed to protect me?” she told me. “And now we’re in the middle of a pandemic and where are they?””
11/ She mailed masks & dozens of records. One batch, apparently bought on Amazon, had skewed markings claiming they’d passed muster with European regulators (“CE”) as if applied by hand with an ink stamp rather than precise machinery.
12/ In a letter in early July, @OSHA_DOL told the company that regulators wouldn’t investigate Steadman’s complaint. Instead it deferred to the company to investigate itself. CSL responded - all good, nothing to see here.
13/ OSHA was apparently satisfied with CSL’s response and closed the case. But not before CSL provided some interesting documents about the masks it gave employees ...
14/ CSL sent a “technical data sheet,” which it says came from a medical supply vendor. It purports to show masks distributed to employees across the country had been tested by a global inspection company and were highly effective. But I’d seen these sorts of documents before …
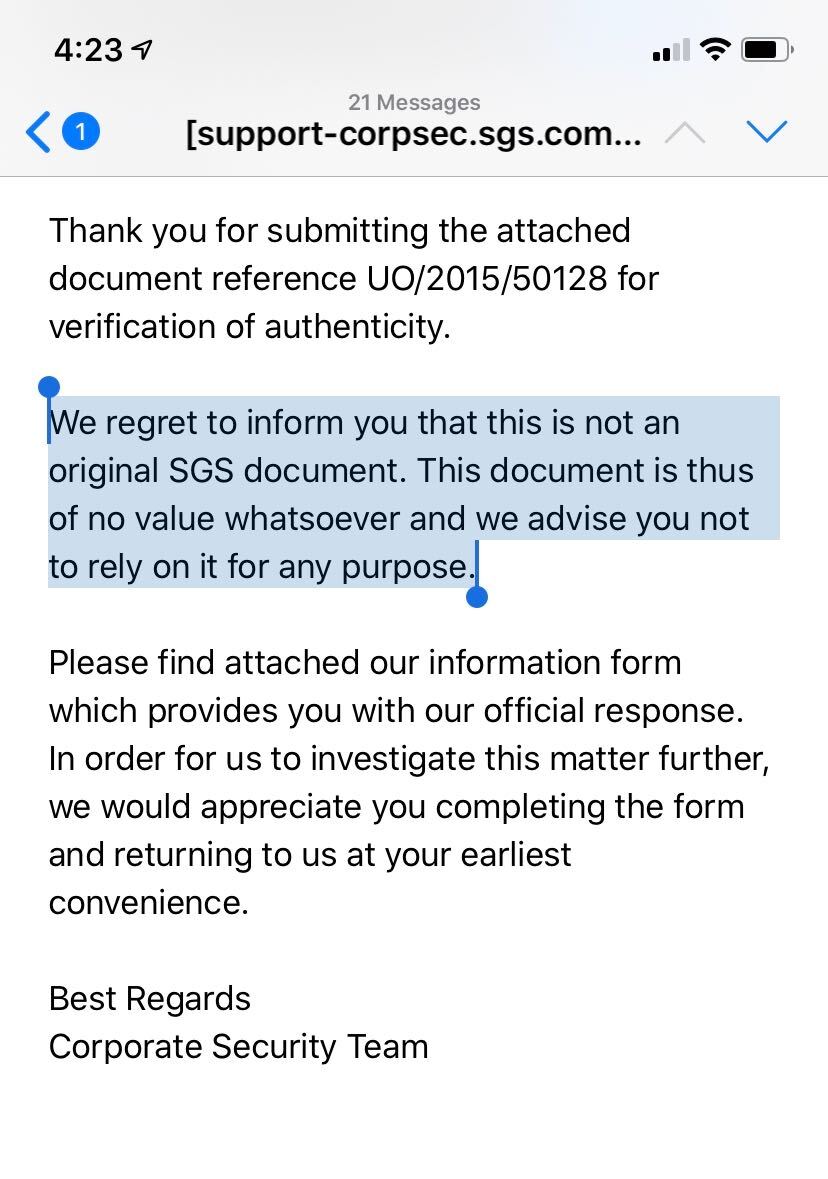
15/ The document was allegedly created by SGS, a global firm that deploys inspectors to factories to test goods for far-flung buyers. But the date followed an American date convention - month, day, year - which I’d not seen on other SGS reports …
16/ And it pertained to a completely different mask. For more answers, I sent the documents to SGS and an expert - a broker who had imported and sold legitimate Chinese-made PPE to government agencies. That broker said he would not buy these masks …
17/ SGS told him the documents supporting CSL’s masks was “a copy and paste job.” The company also told me the document CSL sent @OSHA_DOL was bogus.
18/ I followed up with CSL about the document it gave to OSHA, and the company said it takes its responsibility to give federal regulators true information with “utmost seriousness” and looked into it …
19/ Meanwhile, I noticed something odd. That mask, which was definitely not on the FDA’s list of authorized KN95s throughout my reporting, was suddenly there!
BeforeAfter
BeforeAfter
20/ FDA officials told me this brand of mask was added just days after I asked CSL about the masks. The FDA hadn’t tested the masks themselves but relied on documents that showed its European counterpart approved the mask (the mask Steadman got lacked that “CE” mark)
21/ So how at-risk are the people drawing blood plasma for our president’s shot at a miracle cure before an history election? Potentially very, experts told me.
22/“If I were working in this type of facility, I would like to have an N95 mask,” @T_Inglesby told me.
“You’d have to call these people healthcare professionals, for sure. They’re drawing blood.”
“You’d have to call these people healthcare professionals, for sure. They’re drawing blood.”
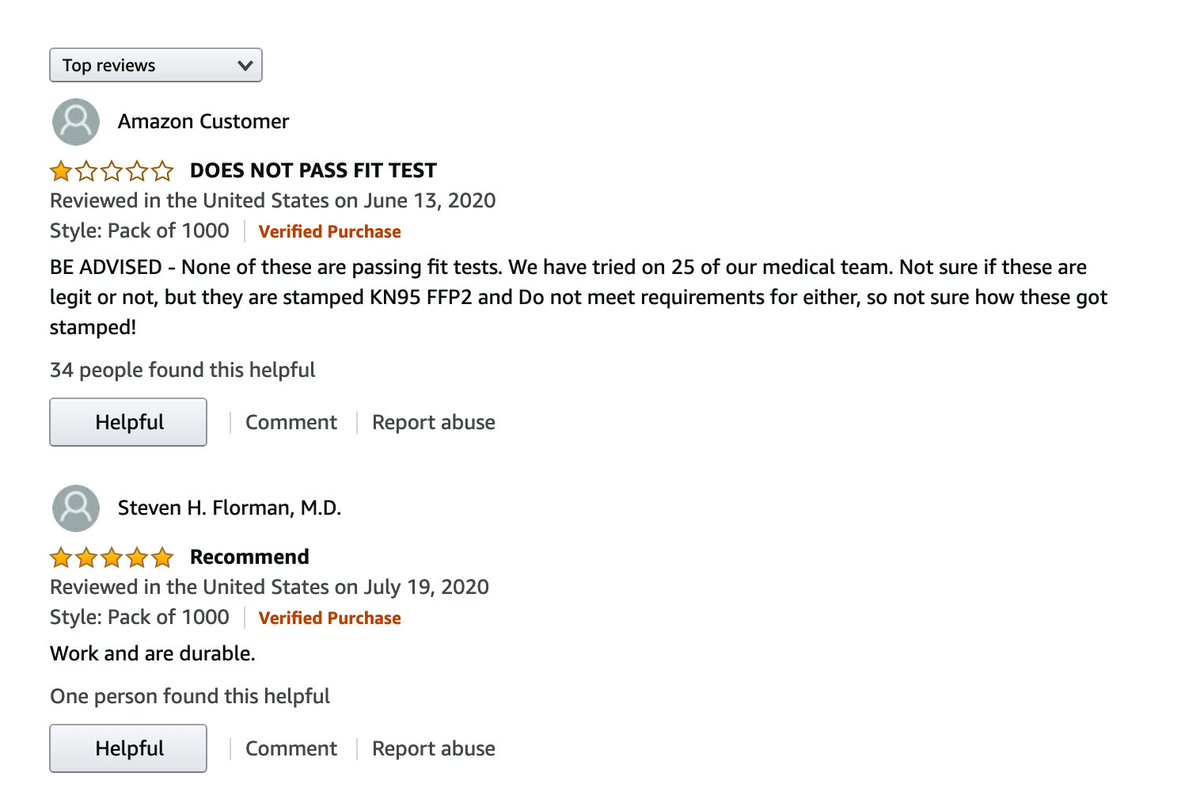
23/ CSL provided other Chinese masks, some tracing back to a diaper factory, others apparently purchased on Amazon. The top review is not great ..
24/ The company eventually sent me some new records, including a testing document and a letter apparently from a ZK-Best manager named Chensu who says this was just a mixup and the masks are good … (factory did not respond)
25/ The company declined to show proof this letter came from the factory. The factory didn’t respond. Steadman says she’s skeptical and her trust in her employee is “unrepairable.” She’s not going back.
26/ Steadman’s story shows the fear wrought by lax government oversight of the Coronavirus mask market, where intense demand and low supply bred graft and fraud and has everyday Americans placing faith in face coverings that might work, maybe not -- who knows? ¯\_(ツ)_/¯
27/ And it highlights the confusion playing out as three Trump administration missteps collide:
Mixed messaging by the White House and CDC
FDA clearing untested masks early on in the pandemic
@OSHA_DOL asleep at the wheel
Mixed messaging by the White House and CDC
FDA clearing untested masks early on in the pandemic
@OSHA_DOL asleep at the wheel
28/ Yes, I once again fell into the maddening vortex that is the absurd PPE market. There’s tons more detail in the story. I hope you’ll read it … https://www.propublica.org/article/foreign-masks-fear-and-a-fake-certification-staff-at-csl-plasma-say-conditions-at-donation-centers-arent-safe">https://www.propublica.org/article/f...
29/ If you want to get our next big stories, sign up for propublica’s Big Story newsletter: http://go.propublica.org/bigstory-social ">https://go.propublica.org/bigstory-...

 Read on Twitter
Read on Twitter