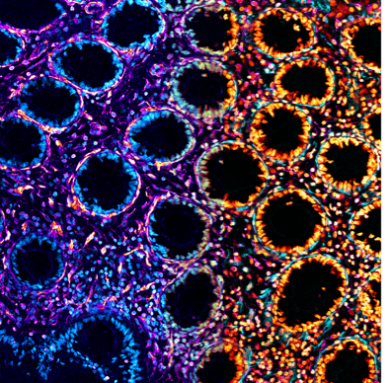
Delighted to share our paper out today in @NatureMaterials where we show that type-1 #innate lymphoid cells (ILC1) drive gut epithelial & matrix remodelling through TGFβ1&MMP9, which has implications for patients with inflammatory bowel disease (IBD). A https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://www.nature.com/articles/s41563-020-0783-8">https://www.nature.com/articles/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread"> https://www.nature.com/articles/s41563-020-0783-8">https://www.nature.com/articles/...
ILC1 are rare immune cells that reside at mucosal barriers. They respond quickly and antigen-non-specifically to viral infections. They also accumulate in the inflamed intestinal tissues of IBD patients, but it’s unclear whether they respond to damage or drive pathology. (2/25)
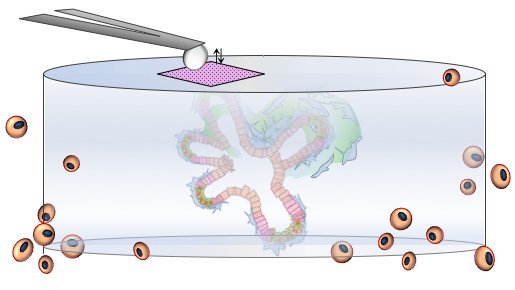
To better understand the impact of ILC1 accumulation in the gut, we created both co-cultures of murine intestinal organoids with lamina propria ILC1, and human #iPSC -derived intestinal #organoids with ILC1 derived from IBD patient biopsies. (3/25)
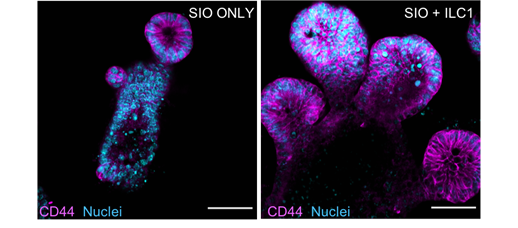
We found that ILC1 unexpectedly drove growth of enlarged CD44+ epithelial #stem cell crypts. This was mediated by ILC1-derived TGFβ1, which specifically upregulated CD44 splice variant 6 across the entire crypt. (see also brilliant https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">from 1st author @GeraldineJowett
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">from 1st author @GeraldineJowett https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)
We were then able to use this #NC3Rs friendly reductionist model to find that a p38γ/β-catenin mechanism drove the epithelial proliferation. ( https://abs.twimg.com/emoji/v2/... draggable="false" alt="😎" title="Lächelndes Gesicht mit Sonnenbrille" aria-label="Emoji: Lächelndes Gesicht mit Sonnenbrille">, nice organoid)(5/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😎" title="Lächelndes Gesicht mit Sonnenbrille" aria-label="Emoji: Lächelndes Gesicht mit Sonnenbrille">, nice organoid)(5/25)
But, were our in vitro organoid cultures in Matrigel relevant to what happens in the intestines of IBD patients? (6/25)
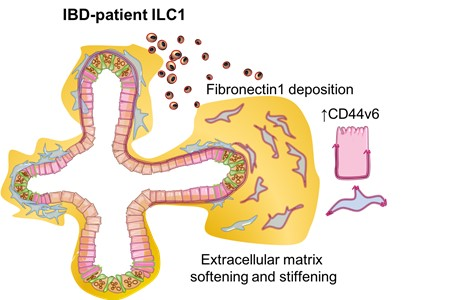
To find out, we looked at sections of IBD patients’ inflamed intestinal tissues and similarly found enlarged CD44v6+ crypts. But, more surprisingly, the CD44v6 was also expressed strongly in the mesenchyme. (7/25)
And when we looked  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔬" title="Mikroskop" aria-label="Emoji: Mikroskop">again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔬" title="Mikroskop" aria-label="Emoji: Mikroskop">again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)
TGFβ1 is known to regulate #fibrosis, which is a common co-morbidity in IBD. We also observed degradation of the Matrigel in our co-cultures ( #fistulae ?). Together, it was starting to look like ILC1 also played a role in extracellular matrix remodelling! (9/25)
But, it’s not possible to study extracellular matrix remodelling when organoids are cultured in Matrigel. (10/25)
We needed a synthetic #hydrogel that was soft like the intestine, but that could form quickly and efficiently https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧪" title="Test tube" aria-label="Emoji: Test tube">. (11/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧪" title="Test tube" aria-label="Emoji: Test tube">. (11/25)
So, we simulated  https://abs.twimg.com/emoji/v2/... draggable="false" alt="💻" title="Computer" aria-label="Emoji: Computer"> gelation of different hydrogel designs using molecular dynamics, which pinpointed a cross-linking strategy that avoided primary looping of the polymer chains. Awesome work by @SuzetteLust, @ChrisLorenz and @CecileDreiss. (12/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💻" title="Computer" aria-label="Emoji: Computer"> gelation of different hydrogel designs using molecular dynamics, which pinpointed a cross-linking strategy that avoided primary looping of the polymer chains. Awesome work by @SuzetteLust, @ChrisLorenz and @CecileDreiss. (12/25)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">
Shout out to Suzie’s mum who wanted to read the proofs and then asked @SuzetteLust: ‘Do you understand all this?’ #PhDlife @AcademicChatter (13/25)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="😆" title="Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😆" title="Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen" aria-label="Emoji: Lächelndes Gesicht mit geöffnetem Mund und fest verschlossenen Augen"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤣" title="Lachend auf dem Boden rollen" aria-label="Emoji: Lachend auf dem Boden rollen"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="😍" title="Lächelndes Gesicht mit herzförmigen Augen" aria-label="Emoji: Lächelndes Gesicht mit herzförmigen Augen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😍" title="Lächelndes Gesicht mit herzförmigen Augen" aria-label="Emoji: Lächelndes Gesicht mit herzförmigen Augen">
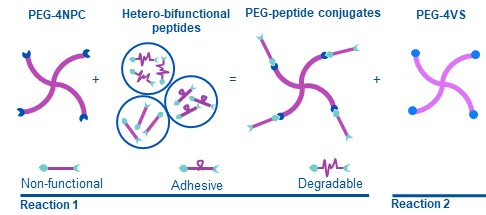
Our modular PEG hydrogel design uses hetero-bifunctional peptide cross-linkers, allowing all PEG arms to participate in cross-linking. Phenomenal effort led by @tracytongyu & @theevanslab, @HooglandScience, @OP_Oommen at @TampereUni and Jons Hilborn at @UU_University (14/25)
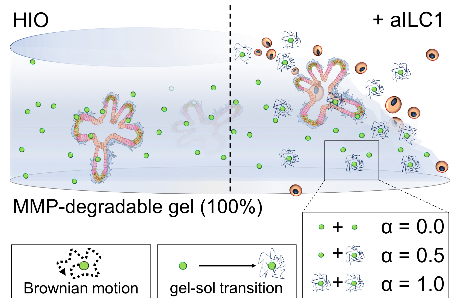
Our new synthetic hydrogel design provided complete and orthogonal control over matrix properties like degradability and stiffness, allowing us to untangle how ILC1 impacted that matrix. (15/25)
Teaming up with @LondonNanotech and @LaurentBozec, PhD student @MichaelNorman used #AFM to show that ILC1 mediated both local softening and stiffening of the hydrogel. (16/25)
Stiffening was mediated by increased expression of fibronectin in the mesenchyme, behaviour that matches what we would expect from a wound healing response. (17/25)
The softening was more difficult to demonstrate. We knew that ILC1 expressed MMP9, which could degrade the hydrogels, but how to show this? (18/25)
We drew inspiration from work by Kelly Schultz and @AnsethGroup (& helpful conversations with @OtgerCampas  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Folded hands" aria-label="Emoji: Folded hands">) and monitored ILC1-driven local hydrogel degradation using multiple particle tracking microrheology https://www.pnas.org/content/112/29/E3757)">https://www.pnas.org/content/1... (19/25)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Folded hands" aria-label="Emoji: Folded hands">) and monitored ILC1-driven local hydrogel degradation using multiple particle tracking microrheology https://www.pnas.org/content/112/29/E3757)">https://www.pnas.org/content/1... (19/25)
Lovely piece of work by @EvaHamrud. Her R code is free to download at https://github.com/eileengentleman/Microrheology-code.">https://github.com/eileengen... (20/25)
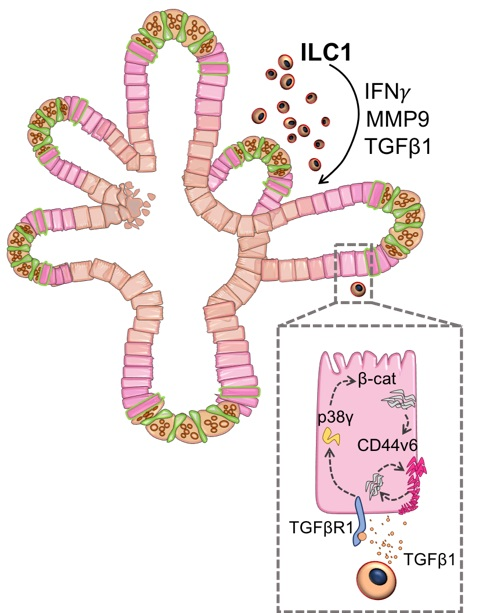
So, in summary, ILC1 secrete TGFβ1, driving proliferation of CD44v6+ epithelial crypts, as well as extracellular matrix remodelling (also through IBD biomarker MMP9) when co-cultured with gut organoids. (21/25)
But, what does it all mean? It may be that ILC1 play a tissue reparative role in the gut, but that their accumulation in inflamed tissues could increase the risk for IBD patients to develop co-morbidities like #fibrosis and #cancer / #tumor growth or #metastasis. (22/25)
There’s so much we still don’t know and so much left for us to explore within the gut human organoid platform @GutOrganoid at @KingsCollegeLon & @kingsdentistry. But, our findings may also have implications beyond IBD. (23/25)
For example, ILC1 are 1st responders to viral infections in the lung & gut. Even asymptomatic patients infected with SARS-CoV2 can show signs of lung fibrosis. So, it’s possible that ILC1 may also accumulate & play a role in driving lung fibrosis in #COVID19 patients. (24/25)
Thanks to our funders at @wellcometrust, @The_MRC, @BBSRC, @UKRI_News, and to the @CSCRM and @CCRB27 (end)

 Read on Twitter
Read on Twitter
 from 1st author @GeraldineJowetthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)" title="We found that ILC1 unexpectedly drove growth of enlarged CD44+ epithelial #stem cell crypts. This was mediated by ILC1-derived TGFβ1, which specifically upregulated CD44 splice variant 6 across the entire crypt. (see also brillianthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">from 1st author @GeraldineJowetthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)" class="img-responsive" style="max-width:100%;"/>
from 1st author @GeraldineJowetthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)" title="We found that ILC1 unexpectedly drove growth of enlarged CD44+ epithelial #stem cell crypts. This was mediated by ILC1-derived TGFβ1, which specifically upregulated CD44 splice variant 6 across the entire crypt. (see also brillianthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">from 1st author @GeraldineJowetthttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🤳" title="Selfie" aria-label="Emoji: Selfie">) (4/25)" class="img-responsive" style="max-width:100%;"/>
 , nice organoid)(5/25)" title="We were then able to use this #NC3Rs friendly reductionist model to find that a p38γ/β-catenin mechanism drove the epithelial proliferation. (https://abs.twimg.com/emoji/v2/... draggable="false" alt="😎" title="Lächelndes Gesicht mit Sonnenbrille" aria-label="Emoji: Lächelndes Gesicht mit Sonnenbrille">, nice organoid)(5/25)" class="img-responsive" style="max-width:100%;"/>
, nice organoid)(5/25)" title="We were then able to use this #NC3Rs friendly reductionist model to find that a p38γ/β-catenin mechanism drove the epithelial proliferation. (https://abs.twimg.com/emoji/v2/... draggable="false" alt="😎" title="Lächelndes Gesicht mit Sonnenbrille" aria-label="Emoji: Lächelndes Gesicht mit Sonnenbrille">, nice organoid)(5/25)" class="img-responsive" style="max-width:100%;"/>
 again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)" title="And when we looked https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔬" title="Mikroskop" aria-label="Emoji: Mikroskop">again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)" class="img-responsive" style="max-width:100%;"/>
again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)" title="And when we looked https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔬" title="Mikroskop" aria-label="Emoji: Mikroskop">again at our co-cultures of patient-derived ILC1 and human iPSC-derived intestinal organoids, CD44v6 was also upregulated in the organoid-associated fibroblasts. (8/25)" class="img-responsive" style="max-width:100%;"/>