NEW—Preliminary results from Russian trials find that #COVID19 vaccine candidates led to no serious adverse events and elicit antibody response https://hubs.li/H0vGWRW0
Thread">https://hubs.li/H0vGWRW0&... (1/8)
Thread">https://hubs.li/H0vGWRW0&... (1/8)
The new paper reports the findings of two open-label, non-randomised phase 1/2 trials looking at a frozen formulation and a freeze-dried formulation of a two-part #COVID19 vaccine (2/8)
The two-part #COVID19 vaccine included two adenovirus vectors – recombinant human adenovirus type 26 (rAd26-S) and recombinant human adenovirus type 5 (rAd5-S) (3/8)
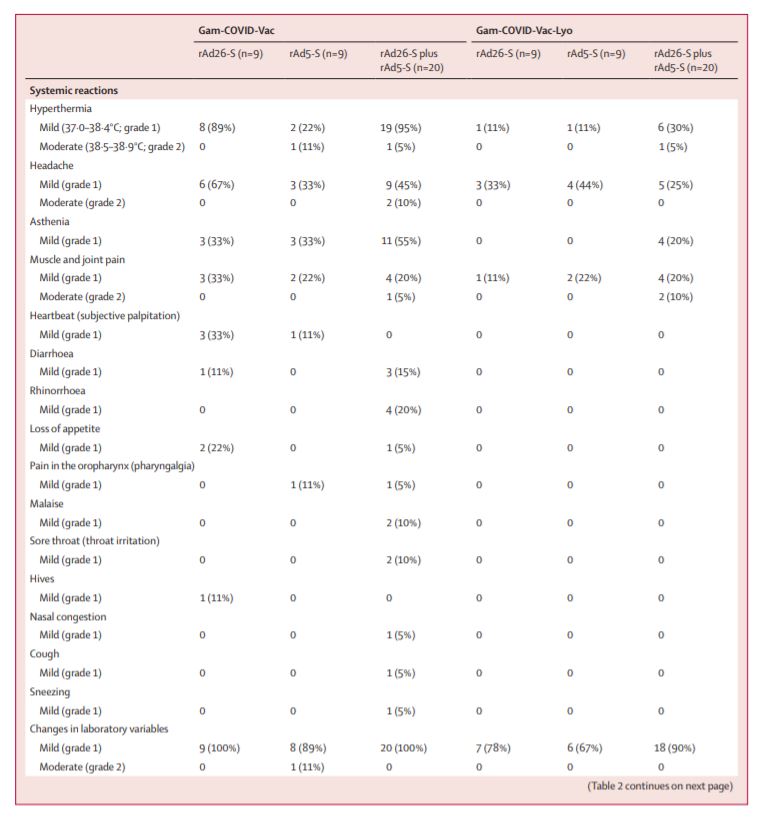
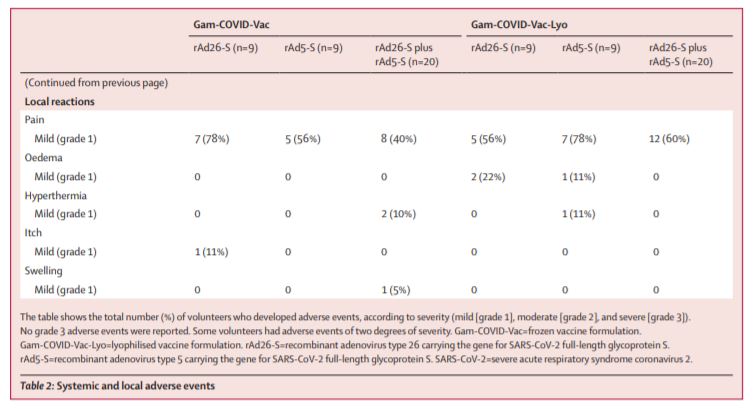
In phase 1 of each trial, individual components of the two-part vaccine (rAd26-S & rAd5-S) were tested for safety
Phase 2 tested whether the vaccine elicited an immune response by giving the full two-part vaccine – rAd26-S was given first, then rAd5-S was given 21 d later (4/8)
Phase 2 tested whether the vaccine elicited an immune response by giving the full two-part vaccine – rAd26-S was given first, then rAd5-S was given 21 d later (4/8)
The two 42-day trials – including 38 healthy adults each – did not find any serious adverse effects among participants (5/8)
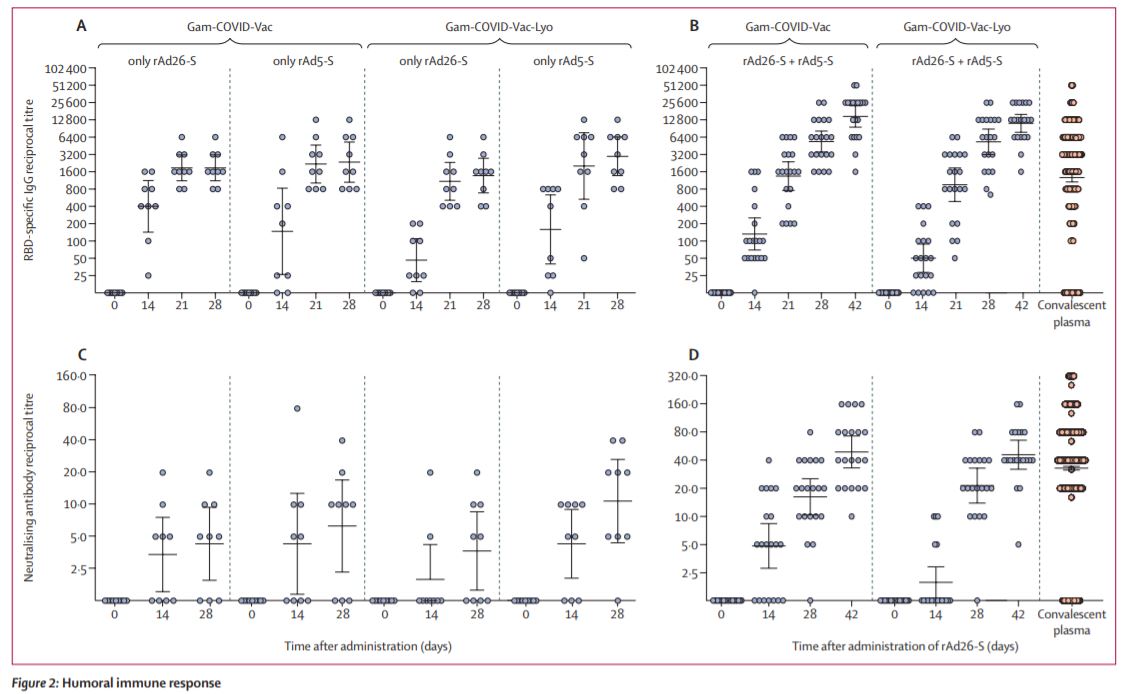
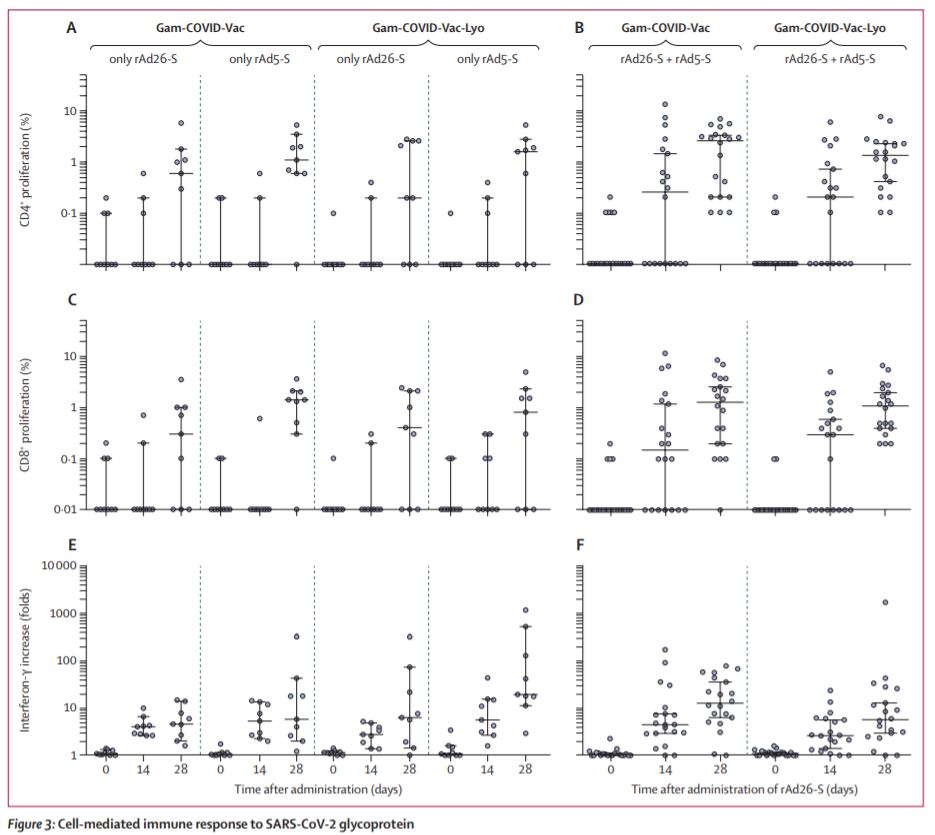
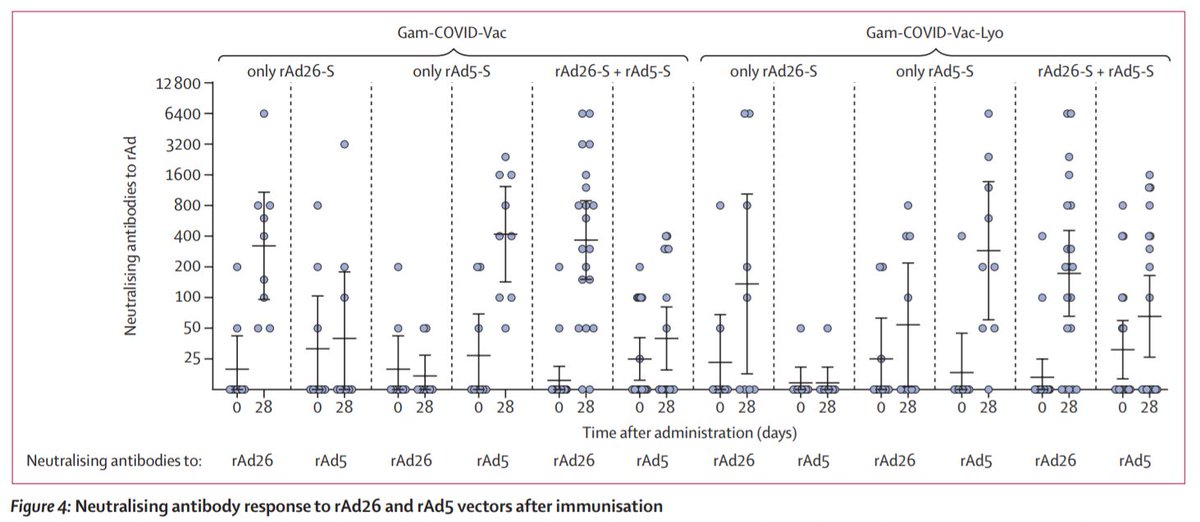
Secondary outcomes from the trial also suggest the vaccines also produce a T cell and neutralising antibody response within 28 days (7/8)
Large, long-term trials including a placebo comparison and further monitoring, are needed to establish the long-term safety and effectiveness of the vaccine for preventing #COVID19 infection (8/8) http://hubs.li/H0vGWRW0 ">https://hubs.li/H0vGWRW0&...
In a Comment, @naorbz & @T_Inglesby describe the studies as "encouraging but small. The immunogenicity bodes well, although nothing can be inferred on immunogenicity in older age groups & clinical efficacy for any #COVID19 vaccine has not yet been shown"
https://hubs.li/H0vH9hB0 ">https://hubs.li/H0vH9hB0&...
https://hubs.li/H0vH9hB0 ">https://hubs.li/H0vH9hB0&...

 Read on Twitter
Read on Twitter