News came out today that the solid-state battery company #QuantumScape is going public with a valuation of $3.3B, backed by investors such as Bill Gates and VW. Little is known about their technology though, so here is a recap of what I found. https://www.cnbc.com/2020/09/03/bill-gates-backed-ev-battery-supplier-to-go-public-through-spac-deal.html">https://www.cnbc.com/2020/09/0... #battchat
Their main claim is that they have achieved a functional "anodeless" battery using a solid-state electrolyte. By anodeless they don& #39;t mean that the battery uses no anode, but that the anode is formed in-situ with lithium coming out of the cathode during the first charge.
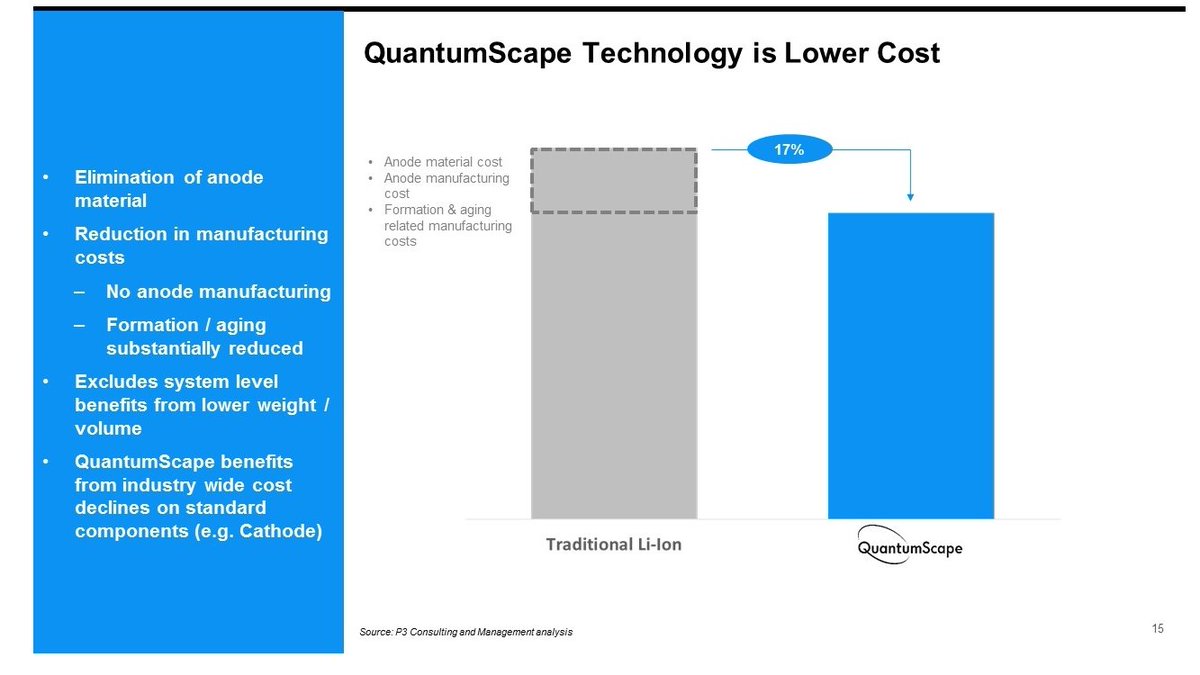
This anodeless configuration would allow them to fabricate lithium metal batteries without the hurdles of using metallic lithium in the manufacturing process, therefore saving costs.
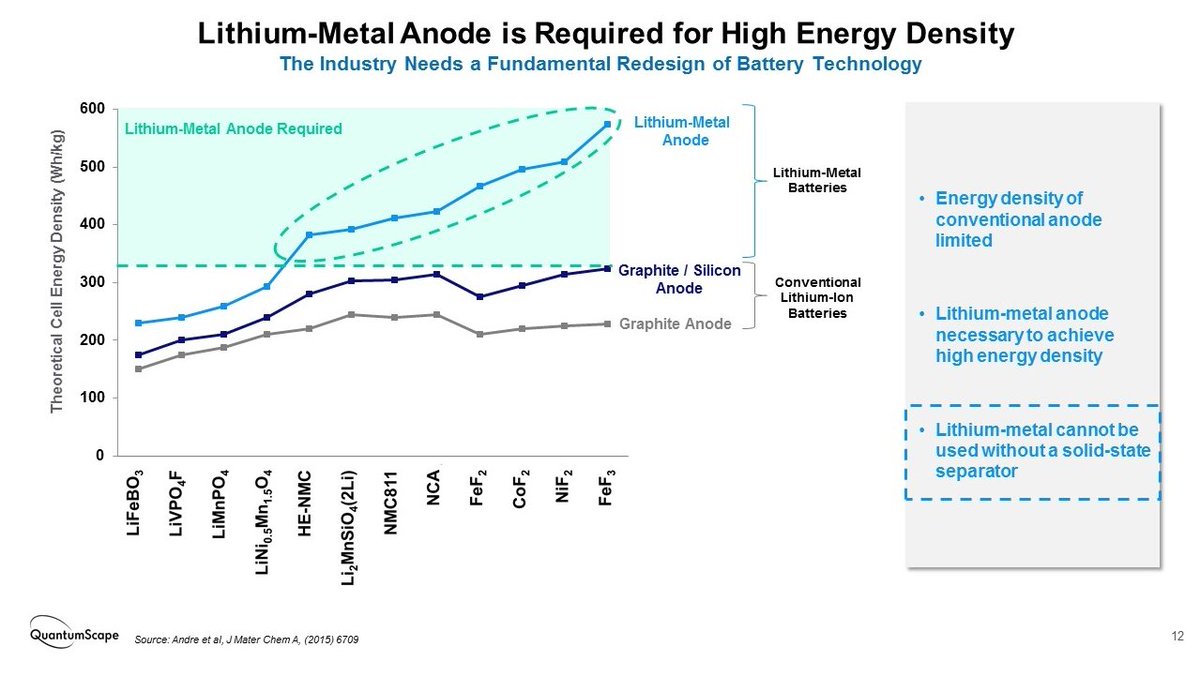
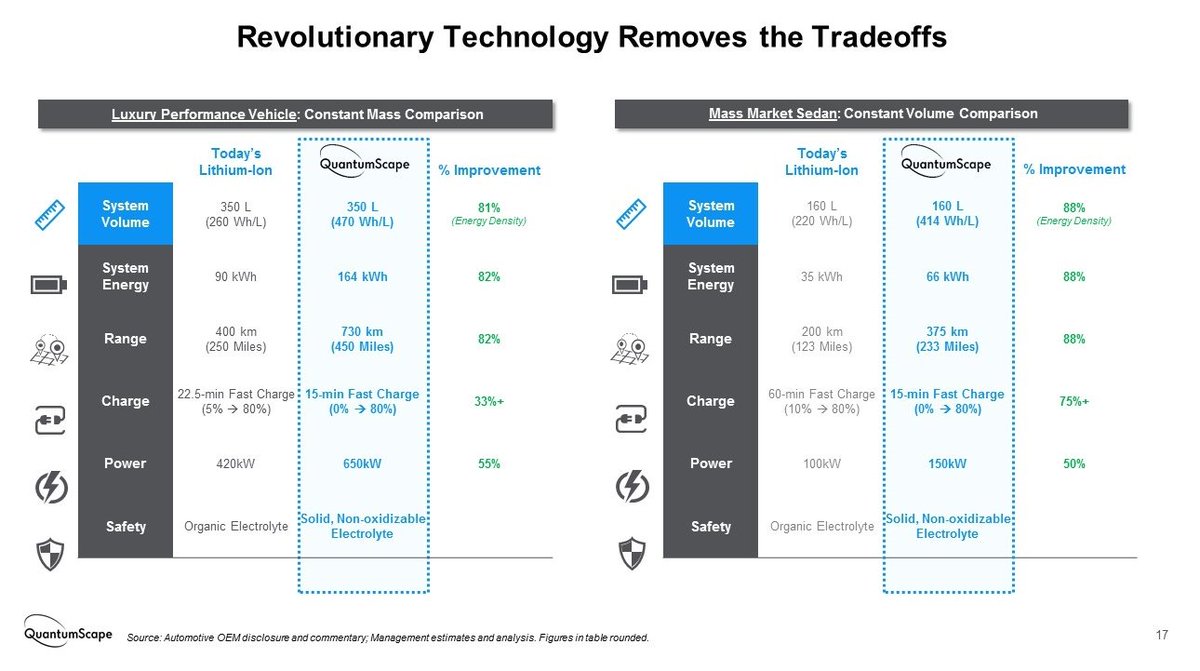
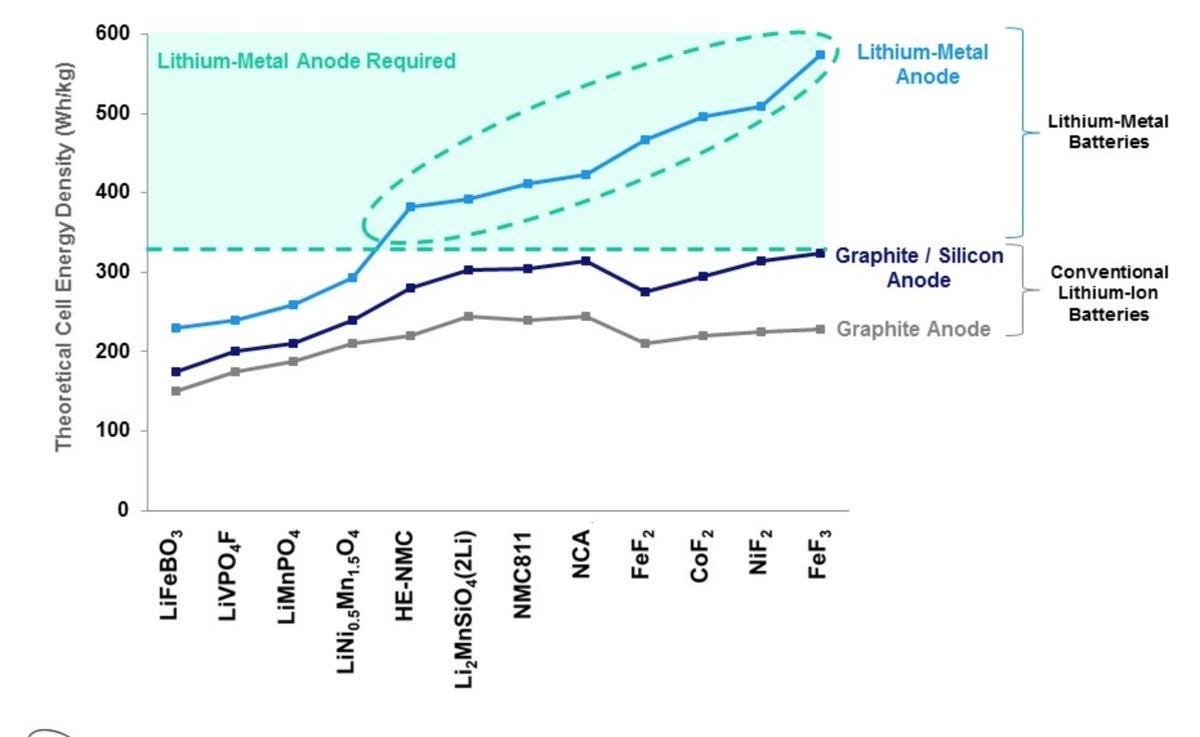
And although no initial lithium metal is present, the in-situ formed anode would give their batteries the high energy densities expected from a lithium anode. Therefore the more than 80% increase in capacity with respect to conventional graphite Li-ion batteries that they claim.
The use of lithium metal as anode has been long sought as a way to significantly boost the energy density of Li-ion batteries. But the high reactivity and dendritic growth of Li metal make it difficult to safely and reliably implement it in a conventional liquid electrolyte cell.
How has QuantumScape managed to get a lithium metal anode battery working with the performance that they claim? The key is the solid-state electrolyte that they have developed.
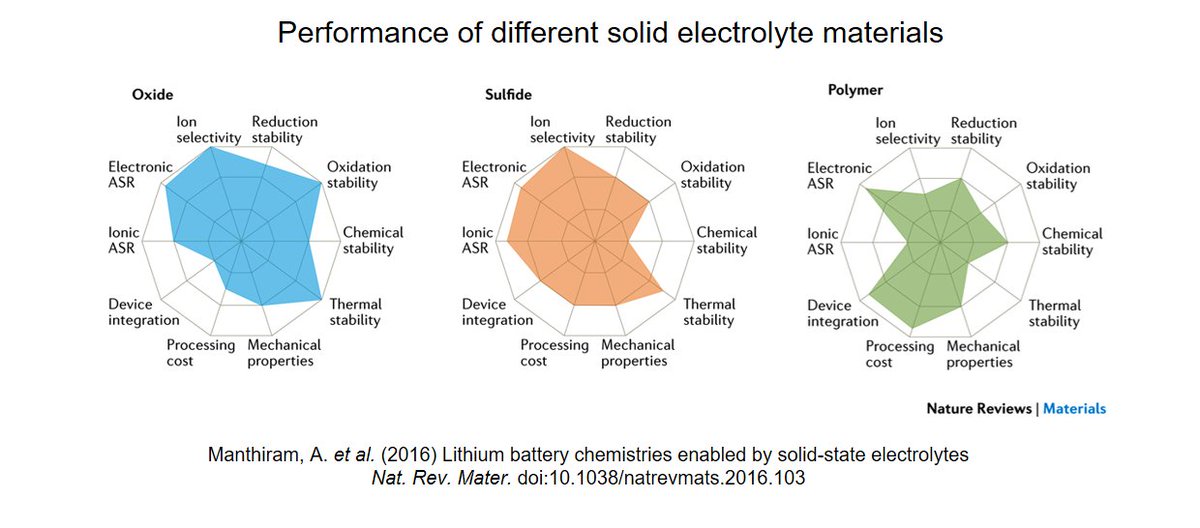
Little information has been made public about the type of solid-state electrolyte they& #39;re using though. If you follow the research on solid-state batteries, you would know that there are three main contestants: polymers, sulfide and oxide electrolytes.
CEO Jagdeep Singh has mentioned that "QuantumScape& #39;s lithium-metal battery uses a solid ceramic electrolyte which he said is safer than a conventional liquid electrolyte". This makes me think that they& #39;re working with some sort of oxide electrolyte, unlike most competitors.
This is actually surprising. Most advanced solid-state batteries presented so far are based on sulfide or polymer electrolytes because they are easier to process and to integrate in a cell. This is the approach of @SolidPowerInc, Toyota, Samsung, Bolloré, Ionic Materials, etc.
Let& #39;s try to find out a bit more what QuantumScape& #39;s solid electrolyte is made of. If we check their patent portfolio, more than 50 of their patents include the words "lithium garnet" in the title, which gives a reasonable clue of what they& #39;re working on. https://patents.justia.com/assignee/quantumscape-corporation">https://patents.justia.com/assignee/...
Lithium garnets are indeed a well-known type of oxide ceramic solid-state electrolytes. Doped Li7La3Zr2O12 (LLZO) is the most promising material within this family, as it has a reasonable high ionic conductivity and is stable against metallic lithium and common cathode materials.
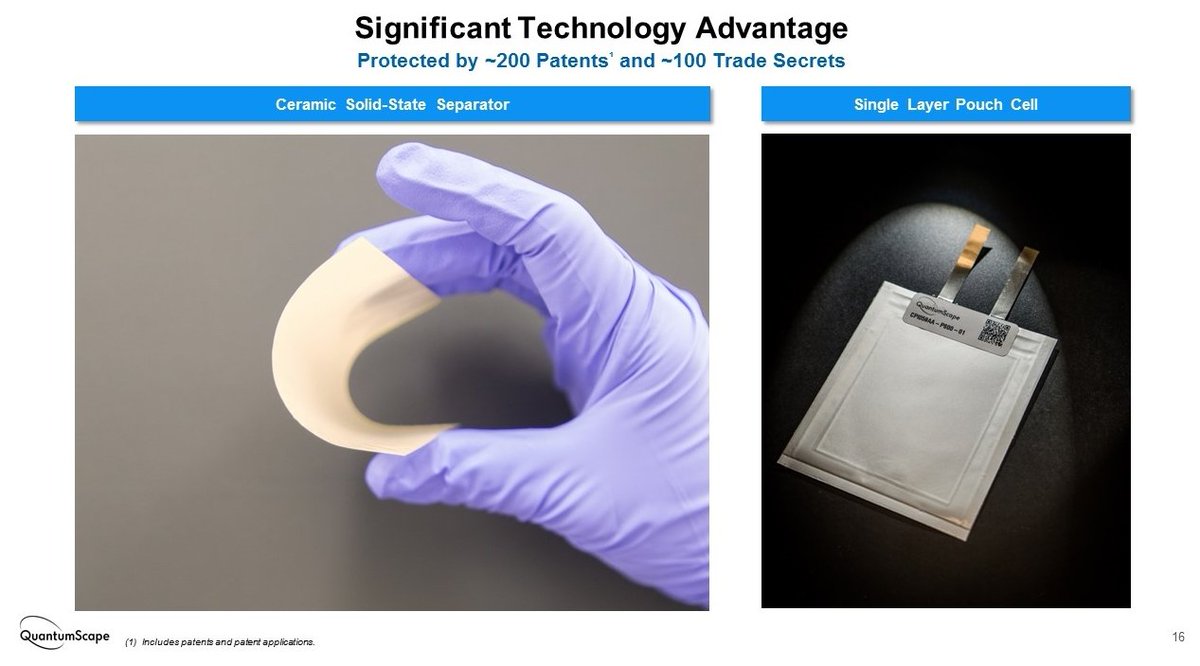
Despite the good electrochemical properties of LLZO, processing this material in a cost-effective and scalable way seemed up to know rather far from feasible. As a ceramic material, it requires high temperature (1200ºC) sintering and it is quite brittle.
It appears that QuantumScape has found a way to fabricate dense LLZO membranes (about 50 um thick) in an industrially feasible approach. This patent gives some details: https://patents.google.com/patent/US10403931B2/en">https://patents.google.com/patent/US...
Besides the manufacturability challenges, LLZO is also known to suffer from lithium dendrite formation at high current densities, especially in thin membranes. QuantumScape must have found a way to mitigate this issue, as in their SEC filing they claim 4C charge rates.
And what about the cathode? Here they seem to be even more ambiguous. In the SEC filing they mention conventional insertion cathodes (e.g. NMC) as well as more advanced conversion-type materials such as iron fluoride.
Given their target energy densities, they seem to be aiming for metal fluoride conversion cathodes. Several of their patents also point towards that direction. https://patents.google.com/patent/US10326135B2
https://patents.google.com/patent/US... href=" https://patents.google.com/patent/US9446966B2
https://patents.google.com/patent/US... href=" https://patents.google.com/patent/EP2816639A2">https://patents.google.com/patent/EP...
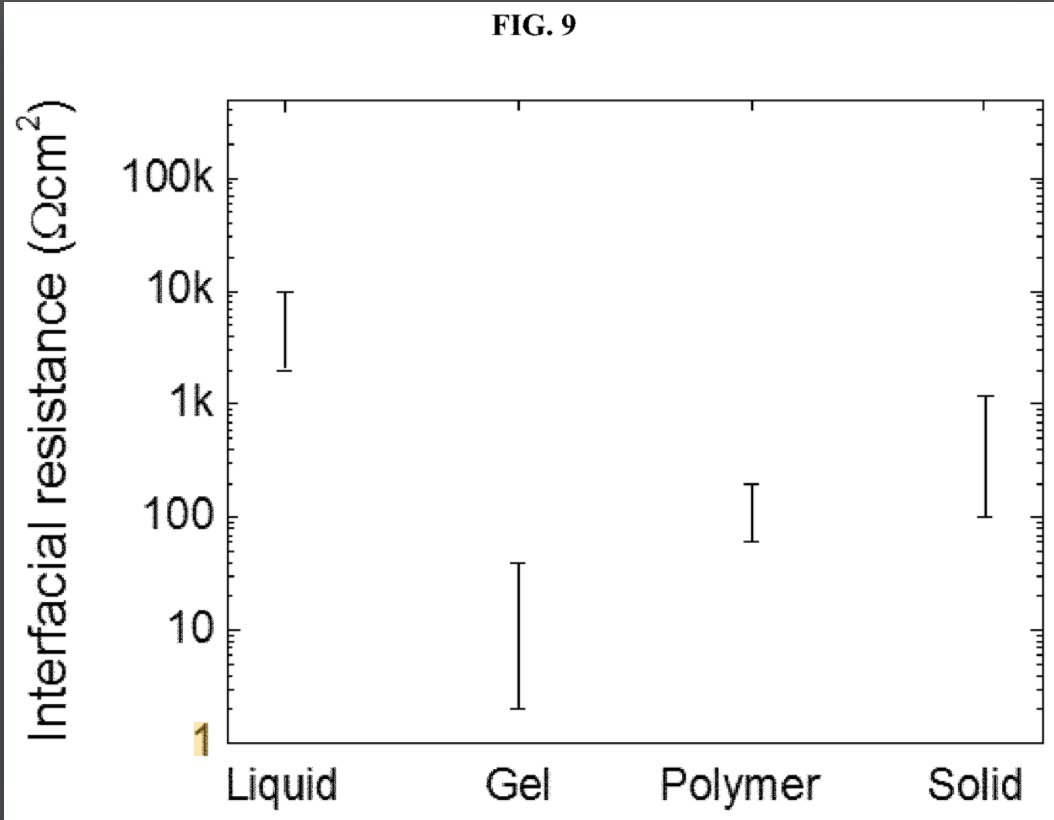
Another intriguing question is how to interface the cathode material with the ceramic separator. Inorganic bonding of LLZO with a cathode material requires high temperature and normally results in a high interfacial resistance as a result of interdiffusion and side reactions.
QuantumScape has a patent on bonding agents for ceramic solid-electrolyte separators. This patent shows gel electrolytes as the most effective bonding agent. This would make their batteries not fully solid state, as the gel contains some solvent. https://patents.google.com/patent/US20200176743A1">https://patents.google.com/patent/US...
Until here my little summary on the technology behind QuantumScape& #39;s solid-state battery. If anyone has further details, please feel free to reply to this thread. @LimitingThe @BatteryBulletin @steingart @stevelevine @ndrewwang @BrianTHeligman

 Read on Twitter
Read on Twitter