1/n For my first tweet (!) excited to share this work by an amazing team of physicians & scientists: https://bit.ly/3jFvuZr
Punchline:">https://bit.ly/3jFvuZr&q... Neutrophils are central to the pathogenesis of severe COVID-19.
Neutrophil granule proteins like Resistin predict critical illness and mortality
Punchline:">https://bit.ly/3jFvuZr&q... Neutrophils are central to the pathogenesis of severe COVID-19.
Neutrophil granule proteins like Resistin predict critical illness and mortality
2/n There is an emerging consensus that damage from the immune system drives the most severe manifestations of COVID-19. Macrophages are an important player; otherwise, the nature of this immune response is still murky. To gain some deeper insights…
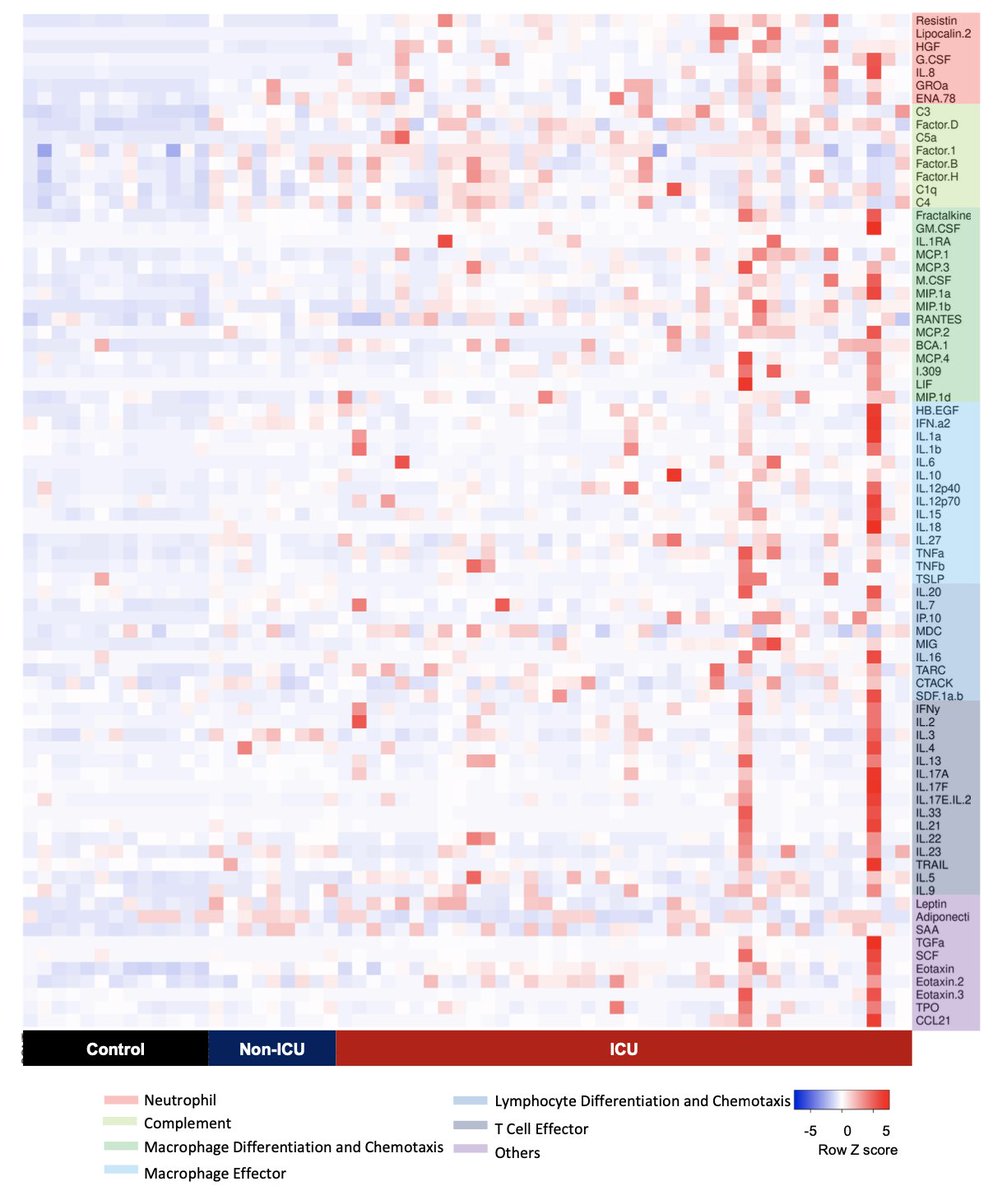
3/n We measured immune proteins in the blood of COVID-19 patients and found, using a machine learning model (JasonBishai @david_van_dijk), that these proteins could distinguish patients who were critically ill (in the ICU) from patients with less severe disease (non-ICU).
4/n Which proteins was the model using to identify critical illness? Here’s the ranked list. The ‘aha’ moment came when we realized that almost all the top markers (in red) were involved in neutrophil activation. And those markers *alone* could identify critically ill patients.
5/n Resistin, lipocalin-2, HGF are all stored in neutrophil granules and released when neutrophils are activated. IL-8 stimulates neutrophil chemotaxis to the site of injury or infection. G-CSF drives neutrophil development. All are much higher in ICU than non-ICU patients.
6/n These neutrophil granule proteins were highly correlated with neutrophil counts (left). We leaned on beautiful single-cell RNAseq from @blish_lab @wilk_aaronj to learn that these markers are transcribed in the ‘developing neutrophil’ population they found in severe COVID-19.
7/n But is neutrophil activation just a byproduct of critical illness? Or could it be driving severe disease? To begin to answer this, we looked at a new cohort of longitudinal patient samples. Was the neutrophil signature present when patients arrived at the hospital?
8/n All the neutrophil activation markers were elevated on day 1, not only in patients admitted directly to the ICU, but also in patients who were initially less sick and only later required ICU transfer. In other words, neutrophil activation came before critical illness.
9/n Looking at this a different way: Resistin levels on admission predicted future transfer to the ICU and mortality. Other markers of neutrophil activation on day 1 were also associated with increased mortality and may outperform established clinical markers. #RestistinCOVID19
10/n Finally, to see if we could generalize these mechanistic insights, we looked at Yale-New Haven Health System’s database of >3,300 patients ( @SimonovSays @methodsmanmd) and found that higher immature and mature neutrophil counts on first CBC predicted worse survival.
11/n The takeaway: There is a strong signature of neutrophil activation that appears before critical illness develops in COVID-19 and may drive severe disease. The neutrophil signature may also serve to identify patients who are at high risk of clinical decompensation.
12/n A model: G-CSF drives neutrophil development (emergency granulopoiesis), and IL-8 stimulates migration to the lung (+other organs?), where neutrophils are activated, release granule proteins, and cause collateral tissue damage, leading to clinical decompensation. @BioRender
13/n There’s much more work to be done. Which cells produce G-CSF and IL-8? Is there a threshold of injury to the lung or vasculature that sets this cascade in motion? Do steroids block neutrophil infiltration? We hope this is a step toward better understanding and therapies.
14/n This was a huge team effort @BenignHeme @hyungjchun AlfredLee
JasonBishai @david_van_dijk @georgegoshuamd @EmilyNadelmann @methodsmanmd @SimonovSays @billdamsky @DelaCruzYaleMed @WonChristine @DeepakAtriMD @jennkwanMDPhD @HaleneLab +others. Thanks to these amazing colleagues
JasonBishai @david_van_dijk @georgegoshuamd @EmilyNadelmann @methodsmanmd @SimonovSays @billdamsky @DelaCruzYaleMed @WonChristine @DeepakAtriMD @jennkwanMDPhD @HaleneLab +others. Thanks to these amazing colleagues
15/n https://bit.ly/3jFvuZr ">https://bit.ly/3jFvuZr&q... Thanks to @YaleMed @YaleIBIO @YaleCardiology @YaleHemOnc @YalePCCSM @YaleIMed @RMedzhitov for your mentorship and guidance @VirusesImmunity @KaminskiMed & many others. Most of all, thank you to the patients. I wish that none of this work was necessary

 Read on Twitter
Read on Twitter