(1/14) Really excited to finally share our work on fragmenting molecules for quantum chemistry torsion scans
https://www.biorxiv.org/content/10.1101/2020.08.27.270934v1.">https://www.biorxiv.org/content/1...
QC torsion scans are used in many force fields as target data to fit MM torsion parameters.
#compchem
https://www.biorxiv.org/content/10.1101/2020.08.27.270934v1.">https://www.biorxiv.org/content/1...
QC torsion scans are used in many force fields as target data to fit MM torsion parameters.
#compchem
(2/14) Why do we need to fragment molecules for QC torsion scans?
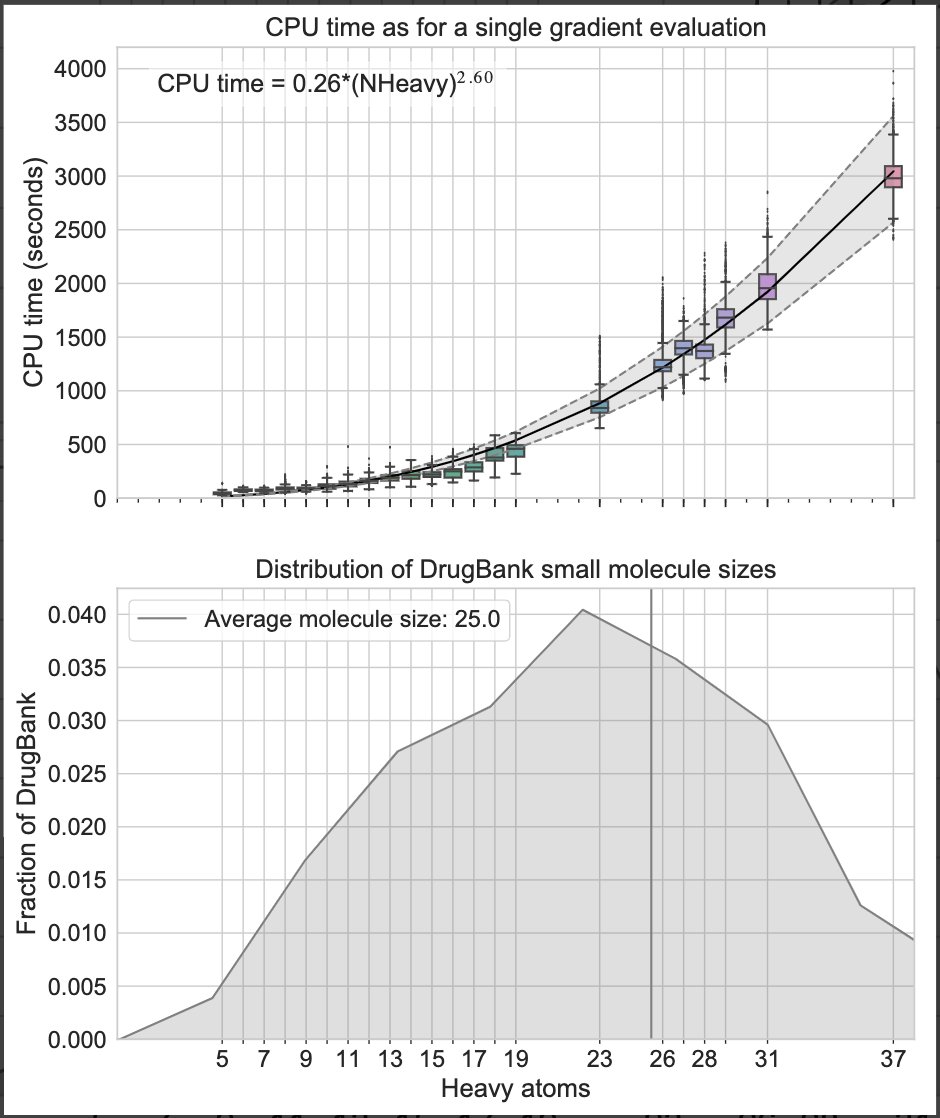
1. QC calculations scale poorly with molecular size. A QC scan for an average FDA approved small molecule drug has 25 heavy atoms takes ~1M CPU s. The cost is reduced by an order magnitude for 15 heavy atom.
1. QC calculations scale poorly with molecular size. A QC scan for an average FDA approved small molecule drug has 25 heavy atoms takes ~1M CPU s. The cost is reduced by an order magnitude for 15 heavy atom.
(3/14) Another reason to fragment molecules is to avoid through-space intramolecular interactions that should be handled by non-bonded terms.
One difference between small molecule and protein ff is the sheer diversity of chemical space small molecule ff need to cover.
One difference between small molecule and protein ff is the sheer diversity of chemical space small molecule ff need to cover.
(4/14) Proteins have natural "building blocks". What are the challenges for creating such building blocks for small molecules?
We want building blocks that retain the chemical environment of the parent molecule. But both local and remote substituents can change that.
We want building blocks that retain the chemical environment of the parent molecule. But both local and remote substituents can change that.
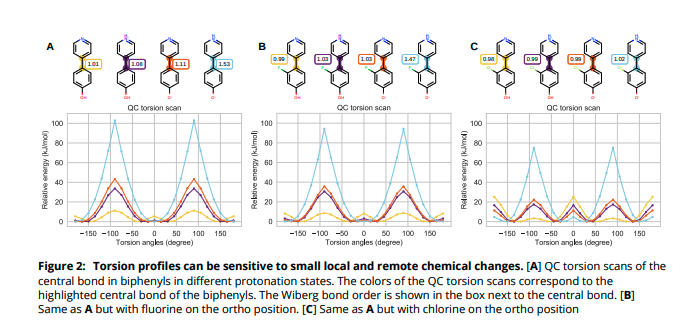
(5/14) We can use the Wiberg bond order (WBO), a measure of electron population overlap across bonds. Turns out, it is sensitive to remote substituent changes that can change the QC torsion profile (see )">https://youtu.be/aRm6c_C8m...
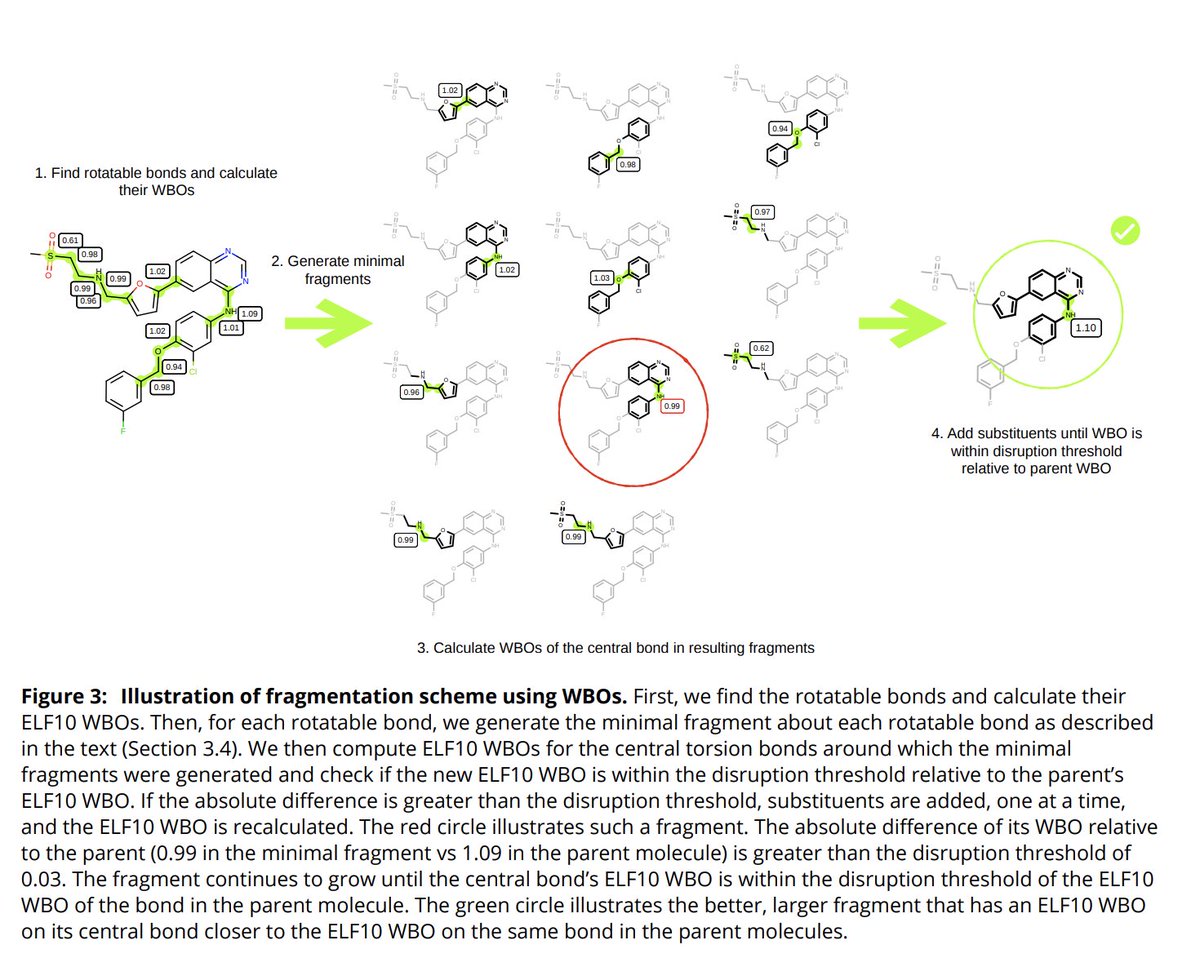
(6/14) We can use the WBO as a cheap surrogate when fragmenting to check if we destroyed the parent& #39;s chemical environment. Our fragmentation scheme builds on the awesome work of Rai et al. https://pubs.acs.org/doi/10.1021/acs.jcim.9b00373">https://pubs.acs.org/doi/10.10...
(7/14) After we find the minimal fragment as described by Rai et. al. we calculate the WBO of the central bond and if the difference with the bond& #39;s parent& #39;s WBO is larger than a threshold, we continue building the fragment.
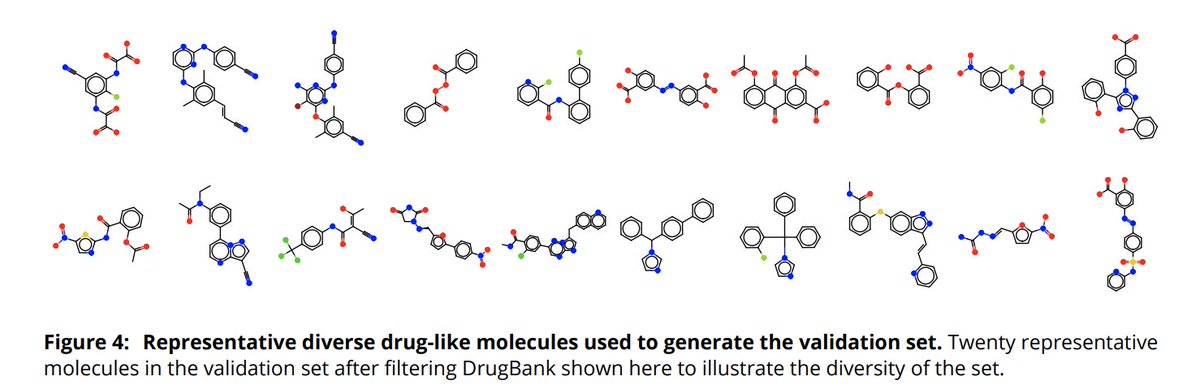
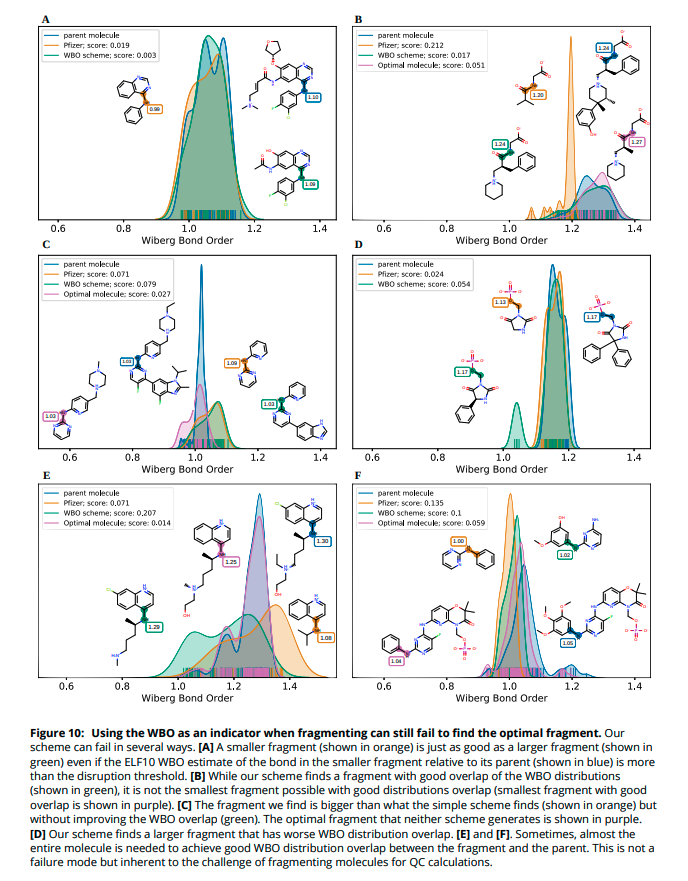
(8/14) How can we verify that this scheme gives us better fragments, especially for molecules that are challenging to fragment? We exhaustively fragmented a diverse subset of drug molecules, generated conformers and calculated the WBO for all bonds in all fragment conformers.
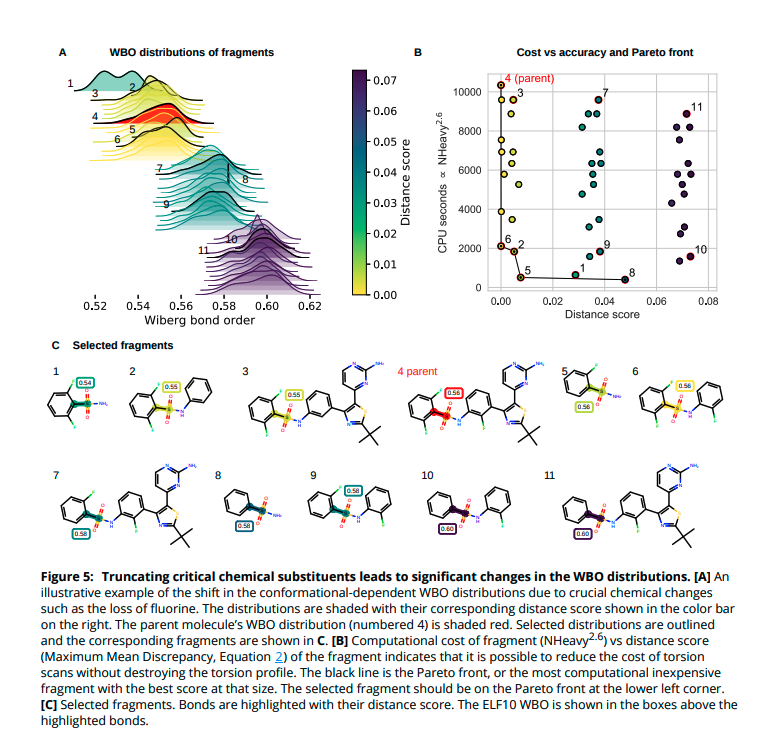
(9/14) The resulting WBO distributions are sensitive to important chemical changes. We can quantify the disruption of the fragment& #39;s chemical environment by calculating the distance of a fragment& #39;s central bond WBO distribution to the WBO distribution of the bond in the parent.
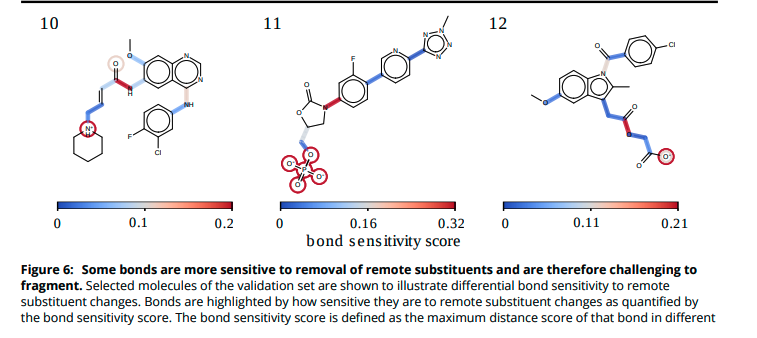
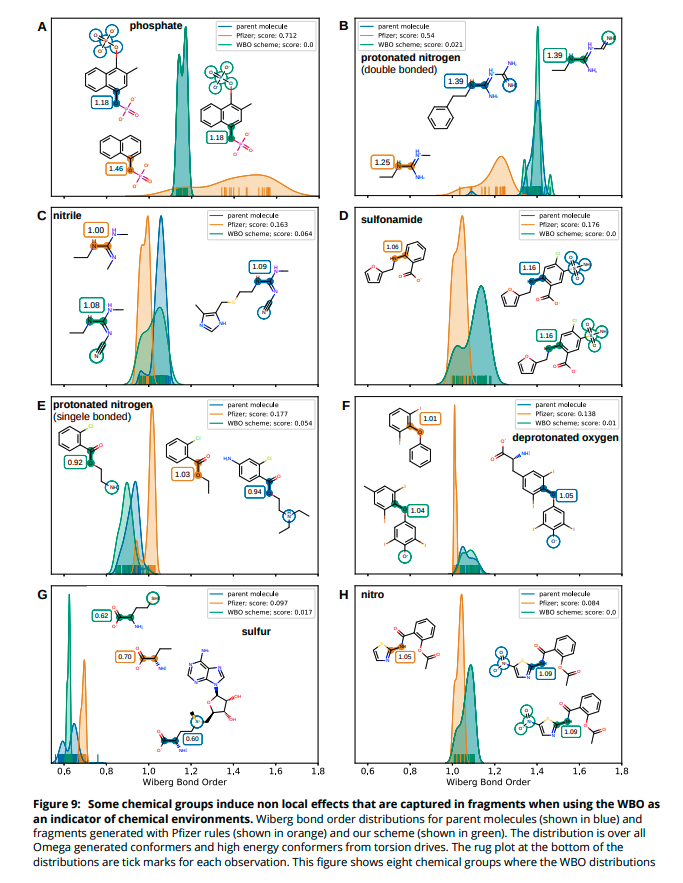
(10/14) Some bonds are more sensitive to remote substituent changes than others. Not surprisingly, conjugated bonds are most sensitive and charged substituents induce the most change.
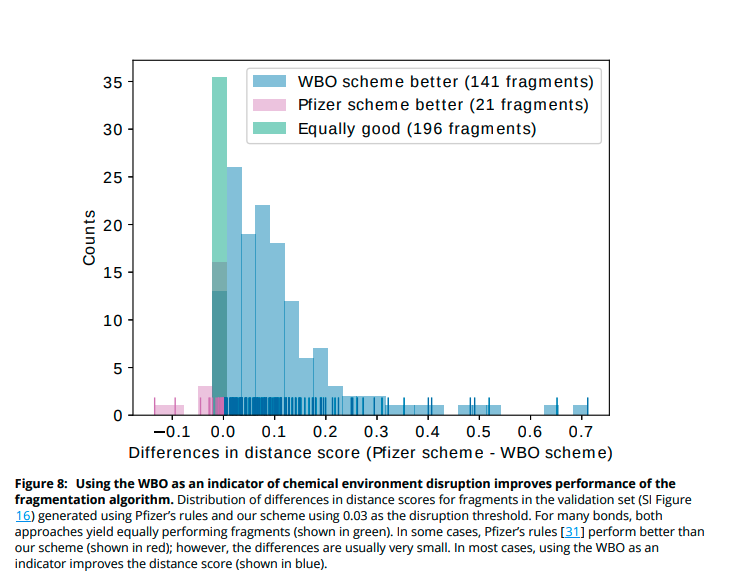
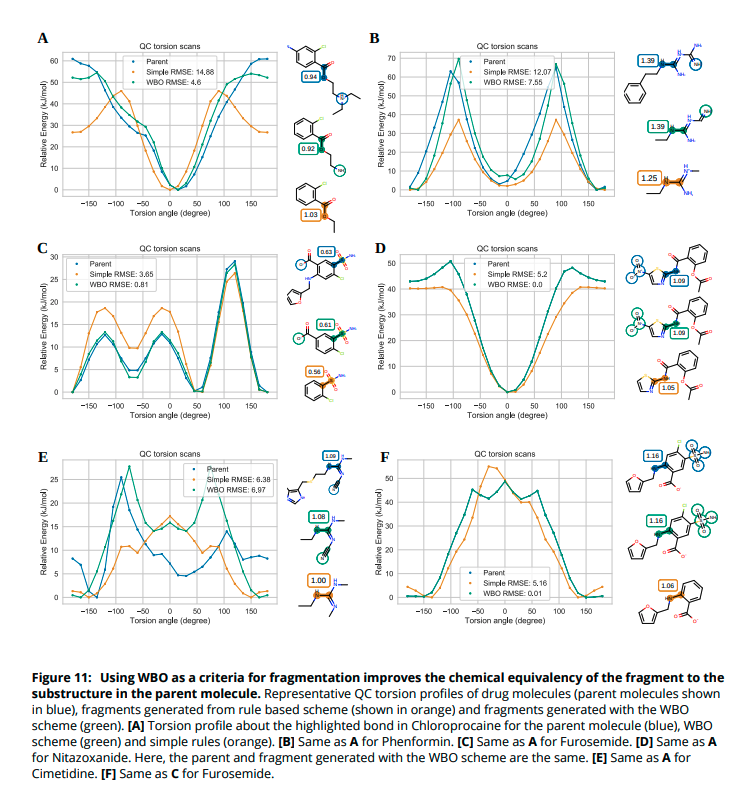
(11/14) We chose a set of 100 molecules that had the most sensitive bonds and fragmented them with our scheme. For most of these molecules, using WBO as an indicator performs better than only using a rule based approach.
(13/14) We ran QC torsion scans for the parent, fragment generated with a rule based scheme and fragments generated using the WBO as an indicator. In the cases we tested, the QC torsion scan was closer to the parent torsion scan when the WBO was used as an indicator.
(14/14) Our scheme sometimes fails to find the optimal fragment - the smallest possible fragment that retains the bonds& #39; parent chemical environment. To always find the optimal fragment, all possible fragments need to be tested which can become too costly.

 Read on Twitter
Read on Twitter