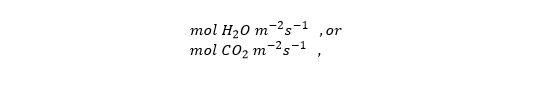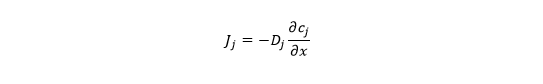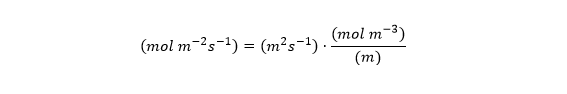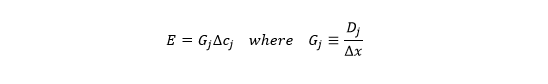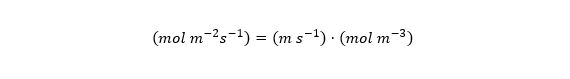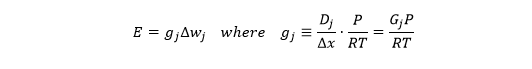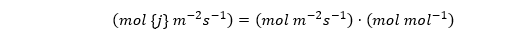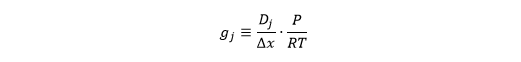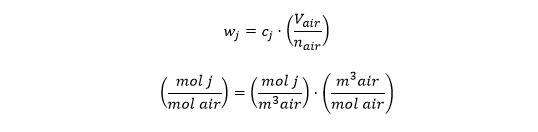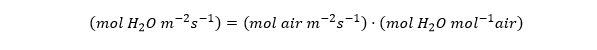1/n
What are the units of stomatal conductance (gs)? The following are often used, apparently on the basis that the conductances for CO2 and H2O differ by a factor of 1.6 (which they do).
But the "H2O" or "CO2" do *not* belong in the units for conductance, and here& #39;s why.
What are the units of stomatal conductance (gs)? The following are often used, apparently on the basis that the conductances for CO2 and H2O differ by a factor of 1.6 (which they do).
But the "H2O" or "CO2" do *not* belong in the units for conductance, and here& #39;s why.
2/n
Where does gs come from? Fick& #39;s First Law says the molar flux of species j (Jj) is proportional to the gradient in its concentration (cj = # of particles per unit volume) and the diffusion coefficient Dx:
Where does gs come from? Fick& #39;s First Law says the molar flux of species j (Jj) is proportional to the gradient in its concentration (cj = # of particles per unit volume) and the diffusion coefficient Dx:
3/n
Dj is commonly given in units of m2 per second. cj has units of moles per m3, and x refers to position along the diffusion path, and has units of m. So the units work out like this:
Dj is commonly given in units of m2 per second. cj has units of moles per m3, and x refers to position along the diffusion path, and has units of m. So the units work out like this:
4/n
If we reverse the sign convention (so x decreases along the forward diffusion path), assume the gradient is constant along the path (turning the partial derivative into a ratio of finite differences), rename J as E, and combine Dj and Delta-x, we get
If we reverse the sign convention (so x decreases along the forward diffusion path), assume the gradient is constant along the path (turning the partial derivative into a ratio of finite differences), rename J as E, and combine Dj and Delta-x, we get
5/n
which looks like the usual equation for transpiration rate (E), with a gradient (Delta-cj) and conductance (Gj). Now the units look like this:
which looks like the usual equation for transpiration rate (E), with a gradient (Delta-cj) and conductance (Gj). Now the units look like this:
6/n
The conductance looks like a velocity (m/s), but I find it helpful to think of it as a volumetric flux (m3 per m2 per s). That makes its relationship to the molar-based conductance clearer (next tweet).
The conductance looks like a velocity (m/s), but I find it helpful to think of it as a volumetric flux (m3 per m2 per s). That makes its relationship to the molar-based conductance clearer (next tweet).
7/n
In plant physiology we prefer to use mole fractions rather than volumetric concentrations for gas diffusion, because this makes the conductance independent of pressure and more weakly dependent on temperature than Dj is by itself (see Cowan 1977). So
In plant physiology we prefer to use mole fractions rather than volumetric concentrations for gas diffusion, because this makes the conductance independent of pressure and more weakly dependent on temperature than Dj is by itself (see Cowan 1977). So
9/n
So the where does mol {j} on the left-hand side come from – the conductance or the mole fraction? It stands to reason that it comes from the mole fraction, which after all is moles of species j per mole of air.
So the where does mol {j} on the left-hand side come from – the conductance or the mole fraction? It stands to reason that it comes from the mole fraction, which after all is moles of species j per mole of air.
10/n
But why *doesn& #39;t* it come from the conductance, even though the conductance somehow "knows" it should be different for H2O and CO2?
To work out the chemical species of the "mol" unit in the conductance, let& #39;s break down gj:
But why *doesn& #39;t* it come from the conductance, even though the conductance somehow "knows" it should be different for H2O and CO2?
To work out the chemical species of the "mol" unit in the conductance, let& #39;s break down gj:
11/n
Are there moles of j hiding in here somewhere? Let& #39;s see. How about Dj – where do the units of Dj come from?
Are there moles of j hiding in here somewhere? Let& #39;s see. How about Dj – where do the units of Dj come from?
12/n
Fick& #39;s First Law can be derived from the kinetic theory of gases. See Atkins& #39; Physical Chemistry text for an excellent example. Dj comes out as being proportional to the mean speed of the diffusing particles (m/s) and the mean free path (m), or m2 * s.
Fick& #39;s First Law can be derived from the kinetic theory of gases. See Atkins& #39; Physical Chemistry text for an excellent example. Dj comes out as being proportional to the mean speed of the diffusing particles (m/s) and the mean free path (m), or m2 * s.
13/n
So the & #39;meters& #39; in Dj refer to spatial dimensions of the system. The mean speed at a given temperature depends on the diffusing species, but what a "meter" means in the context of this system does not. It& #39;s a meter of space, not a meter of species j.
So the & #39;meters& #39; in Dj refer to spatial dimensions of the system. The mean speed at a given temperature depends on the diffusing species, but what a "meter" means in the context of this system does not. It& #39;s a meter of space, not a meter of species j.
14/n
What about P/RT? Does that inject units of mol {j}? Where does P/RT come from anyway? It comes from converting the water vapor concentration (cj) to its mole fraction (wj) by multiplying cj by the molar volume of air (V/n = RT/P from the ideal gas law):
What about P/RT? Does that inject units of mol {j}? Where does P/RT come from anyway? It comes from converting the water vapor concentration (cj) to its mole fraction (wj) by multiplying cj by the molar volume of air (V/n = RT/P from the ideal gas law):
15/n
So the moles introduced into the denominator of the mole fraction are moles of *air*. They replaced the original denominator, which had units of m3 of air.
So the moles introduced into the denominator of the mole fraction are moles of *air*. They replaced the original denominator, which had units of m3 of air.
16/n
But since we multiplied cj by RT/P, we need to multiply Dj by the inverse of RT/P (=P/RT) to avoid changing the overall equation. That means the moles in the conductance term are likewise moles of air, not moles of j:
But since we multiplied cj by RT/P, we need to multiply Dj by the inverse of RT/P (=P/RT) to avoid changing the overall equation. That means the moles in the conductance term are likewise moles of air, not moles of j:
17/n
OK. So then, how do the values of gs for CO2 and H2O "know" they should differ, if the units of gs don& #39;t specify the diffusing species?
They don& #39;t! It& #39;s *we* who know that.
OK. So then, how do the values of gs for CO2 and H2O "know" they should differ, if the units of gs don& #39;t specify the diffusing species?
They don& #39;t! It& #39;s *we* who know that.
18/n
If we could independently measure gs for both CO2 and H2O in the same leaf, we& #39;d find their ratio was about 1.6.
We can& #39;t do that (because we don& #39;t know ci), so we *calculate* gsc from gsw based on *our knowledge* that the diffusion coefficients differ by a factor of 1.6.
If we could independently measure gs for both CO2 and H2O in the same leaf, we& #39;d find their ratio was about 1.6.
We can& #39;t do that (because we don& #39;t know ci), so we *calculate* gsc from gsw based on *our knowledge* that the diffusion coefficients differ by a factor of 1.6.
19/n
So, quod erat demonstradum, post hoc ergo propter kumquat and so forth. It& #39;s clear that the units of conductance are moles of air, not moles of H2O or CO2.
However, comma,...
So, quod erat demonstradum, post hoc ergo propter kumquat and so forth. It& #39;s clear that the units of conductance are moles of air, not moles of H2O or CO2.
However, comma,...
20/n
...if we REALLY want to be anal and follow the rules, we shouldn& #39;t include chemical species anywhere in the units!
From https://physics.nist.gov/cuu/pdf/sp811.pdf:">https://physics.nist.gov/cuu/pdf/s...
...if we REALLY want to be anal and follow the rules, we shouldn& #39;t include chemical species anywhere in the units!
From https://physics.nist.gov/cuu/pdf/sp811.pdf:">https://physics.nist.gov/cuu/pdf/s...
21/n
But if you insist on including chemical species in the units for stomatal conductance, the correct species is "air", not "H2O" or "CO2". The H2O and CO2 go in the mole fraction gradient.
But if you insist on including chemical species in the units for stomatal conductance, the correct species is "air", not "H2O" or "CO2". The H2O and CO2 go in the mole fraction gradient.

 Read on Twitter
Read on Twitter