I spoke to FDA head @SteveFDA about the decision to issue an EUA (emergency use authorization) for convalescent plasma to treat those currently infected. While promising, it’s been controversial because many say the data is inadequate. (1/11) https://www.cnn.com/2020/08/23/health/covid-19-convalescent-plasma-eua-white-house/index.html">https://www.cnn.com/2020/08/2...
Dr. Hahn told me “he should have better explained the data.” Here’s what he (incorrectly) said on Sunday night: Use of convalescent plasma reduced the risk of death by 35% and that meant if 100 people got coronavirus, 35 would survive. (2/11) https://www.whitehouse.gov/briefings-statements/remarks-president-trump-press-briefing-august-23-2020/">https://www.whitehouse.gov/briefings...
Last night he aimed to clarify that by telling me and then tweeting: “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.” This is a fundamental difference and important to understand. (3/11) https://twitter.com/SteveFDA/status/1298071620414824452?s=20">https://twitter.com/SteveFDA/...
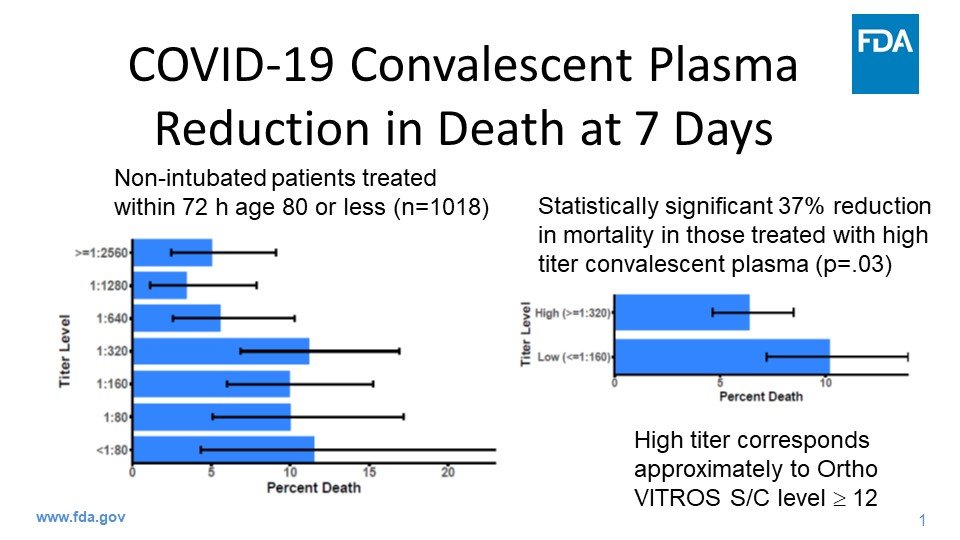
Let me try and explain. According to the Mayo Clinic’s early analysis of over 35,000 hospitalized patients they found that “For patients who received high IgG plasma seven-day mortality was 8.9%... and for recipients of low IgG plasma mortality was 13.7%.” (4/11)
That means the relative risk reduction is calculated like this: (13.7-8.9)/13.7 = .35 or 35%. Those treated with plasma containing the highest levels of antibodies had about a 35% lower risk of dying within a week compared to those treated with less-rich plasma. (5/11)
But, that doesn’t mean what Dr. Hahn originally said: that 35 more people -- out of 100 -- would survive. What that actually means is that 13.7/100 people died using low level antibody plasma, and that 8.9/100 died using the high level antibody plasma. (6/11)
So in the high antibody group, 4.8 more people survived out of 100, as compared to the low antibody group. (7/11)
But, there is another critical point. This was not a randomized controlled trial, the gold standard for clinical trials. Neither group was compared to a placebo, so we still don’t know if the convalescent serum itself offered survival benefit or not. (8/11)
In fact, a closer examination of the study showed 49.8% of the participants were also being treated with steroids, 30.1% were treated with Remdesivir. So, was it the other medications offering the benefit or the convalescent plasma? We can’t say for sure. (9/11)
The study, upon which this EUA was based, hasn’t been peer reviewed or published in a journal. (10/11)
https://www.medrxiv.org/content/10.1101/2020.08.12">https://www.medrxiv.org/content/1...
https://www.medrxiv.org/content/10.1101/2020.08.12">https://www.medrxiv.org/content/1...
Dr. Hahn continued to stand by the EUA tweeting: “On behalf of FDA‘s 18,000 career employees, I want to reassure the American public about this commitment. The convalescent plasma decision was made entirely by FDA scientists.” (11/11) https://twitter.com/SteveFDA/status/1298071616644153345?s=20">https://twitter.com/SteveFDA/...

 Read on Twitter
Read on Twitter