I can& #39;t believe the @US_FDA Commissioner @SteveFDA announced that 35 out of 100 patients treated with #ConvalescentPlasma will benefit from it. This demonstrates either a lack of understanding of basic statistics (relative risk vs absolute risk) or external pressures. (1/n)
There are several problems with this - first, this is not based on randomized evidence. This is based on "data obtained from the ongoing National Expanded Access Treatment Protocol (EAP) sponsored by the Mayo Clinic". The preprint is available on https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1">https://www.medrxiv.org/content/1... (2/n)
In an observational study of 35,322 patients transfused with CP, 7-day mortality was 8.7% in those where CP was transfused early (3 days or less) and 11.9% in those transfused later (>3 days). 30 day mortality was 21.6% vs 26.7%. (3/n)
Important to note that this was an observational comparison of early vs late CP transfusion, not a comparison of CP vs no CP. Being an observational study, it is subject to very high levels of systematic bias. The study also came up with some interesting findings (4/n)
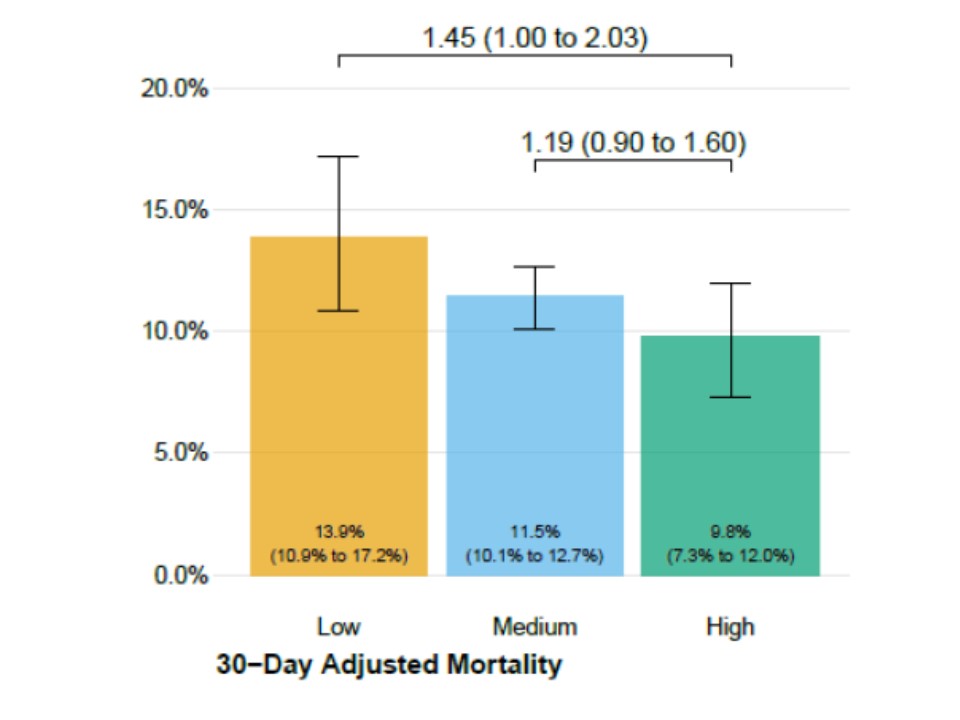
There was graded reduction in mortality depending on the IgG antibody titres of the transfused plasma. 7-day mortality was 8.9% in those receiving high IgG plasma, 11.6% with medium and 13.7% with low IgG plasma. The relative risk of mortality with high vs low IgG CP was 0.65 5/n
Which is the miraculous 35% risk reduction @SteveFDA spoke about. Which is actually only a 4.8% absolute risk reduction. We wrote about the considerations in absolute vs relative risks in https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4763519/
6/n">https://www.ncbi.nlm.nih.gov/pmc/artic...
6/n">https://www.ncbi.nlm.nih.gov/pmc/artic...
Also remember that this 35% risk reduction was not plasma vs no plasma, it wasn& #39;t even early vs late plasma, but a select subgroup of high IgG antibody plasma transfusion with low IgG plasma. Clearly a misleading statement from the @US_FDA Commissioner 7/n
The authors of the EAP, to their credit, acknowledge the limitations of their findings and the fact that the observational study design was a compromise. Why then is this sensational statement of a breakthrough from the @US_FDA so damaging? 8/n
With a public proclamation of a 35% improved survival (which is false), you have effectively killed the possibility of a well-powered randomized trial, which is what is really needed to show whether #ConvalescentPlasma is actually useful in #COVIDー19 9/n
Physicians and patients who are not aware of the ambiguity and serious biases inherent in an observational study and the far more modest benefit (if any) than the touted 35% will henceforth be reluctant to participate in a randomized trial. 10/n
It also seems that the EAP which enrolled >35000 pts (and the total no of close to 70000 pts in the US who have received #ConvalescentPlasma) was a wasted opportunity to do a randomized trial, which would have answered the question conclusively with 1/10 the patients 11/n
Interestingly, @US_FDA site still states "The FDA continues to recommend that the designs of ongoing RCTs of #COVIDー19 convalescent plasma remain unaltered, as COVID-19 convalescent plasma does not yet represent a new standard of care based on current available evidence" 12/n
I hope that #ConvalescentPlasma turns out to be beneficial in randomized trials. The benefit is however likely to be marginal, if at all, and not the 35% improved survival homerun that is being claimed. Yes, a pandemic is still not reason enough to abandon good science. The End

 Read on Twitter
Read on Twitter