Today& #39;s "breakthrough" announcement is unlikely to be any breakthrough.
1. Convalescent plasma has no randomized trials. It contains many antibodies that have no neutralizing effect. If it has efficacy, it will be be relatively modest, at best.
1. Convalescent plasma has no randomized trials. It contains many antibodies that have no neutralizing effect. If it has efficacy, it will be be relatively modest, at best.
2. There are no new, large-scale randomized trials that have been completed with repurposed drugs; the combination of JAK inhibitor (Baricitinib) and Remdesivir may have results but it only has 400 patients and no replication https://investor.lilly.com/news-releases/news-release-details/lilly-begins-phase-3-clinical-trial-baricitinib-hospitalized">https://investor.lilly.com/news-rele...
3. Type 1 interferon (intranasal) early, preventive therapy has considerable promise (reviewed w/ @VirusesImmunity and this https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">; there are many trials but they are in progress
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">; there are many trials but they are in progress
https://twitter.com/EricTopol/status/1293755617807462400
https://twitter.com/EricTopol... href=" https://twitter.com/EricTopol/status/1296935589728538624">https://twitter.com/EricTopol...
https://twitter.com/EricTopol/status/1293755617807462400
4. True breakthroughs in medicine are exceedingly rare.
It is even more rare—actually unprecedented—for they to be announced by a politician, no less a @POTUS
In the context of other magic and cures that have similarly been proclaimed in recent months, this all aligns well.
It is even more rare—actually unprecedented—for they to be announced by a politician, no less a @POTUS
In the context of other magic and cures that have similarly been proclaimed in recent months, this all aligns well.
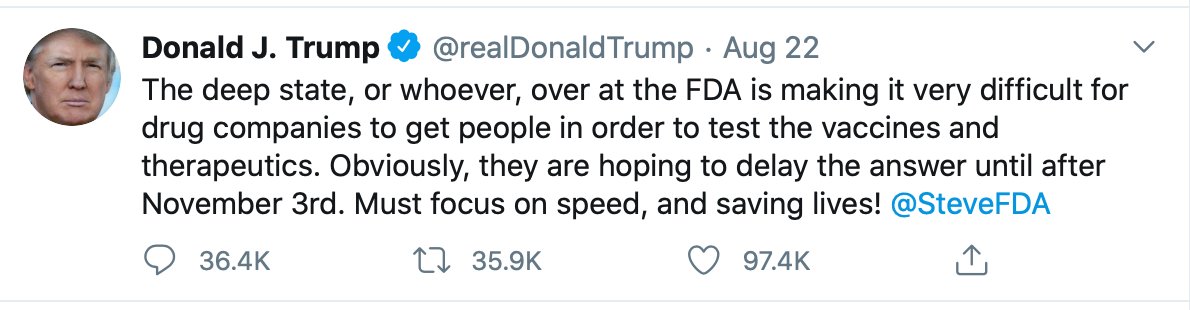
5. It& #39;s even worse than this. There is no breakthrough and the @US_FDA commissioner @SteveFDA has caved to Trump& #39;s pressure (including yesterday& #39;s tweet) to promote it as if there was one.
@statnews @NicholasFlorko
https://www.statnews.com/2020/08/23/fda-under-pressure-from-trump-expected-to-authorize-blood-plasma-as-covid-19-treatment/?utm_content=buffer405b9&utm_medium=social&utm_source=twitter&utm_campaign=twitter_organic">https://www.statnews.com/2020/08/2...
@statnews @NicholasFlorko
https://www.statnews.com/2020/08/23/fda-under-pressure-from-trump-expected-to-authorize-blood-plasma-as-covid-19-treatment/?utm_content=buffer405b9&utm_medium=social&utm_source=twitter&utm_campaign=twitter_organic">https://www.statnews.com/2020/08/2...
Outrageous to listen to @realDonaldTrump @secAzar @SteveFDA claiming a 35% improved survival in an observational preprint study compared w/ late-treated patients. There& #39;s no evidence to support any survival benefit. 2 days ago FDA& #39;s website stated there was no evidence for an EUA
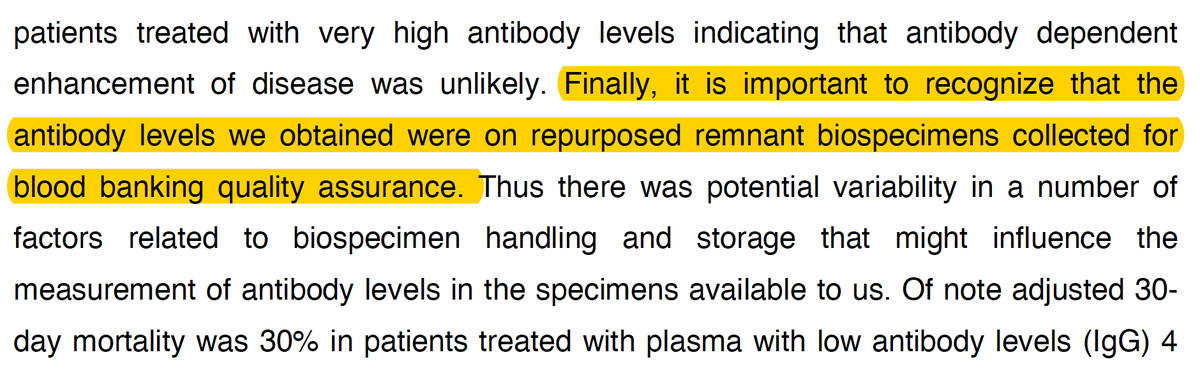
Here& #39;s the 8/12 preprint the claim is based on
https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.full.pdf
These">https://www.medrxiv.org/content/1... were 2 exploratory analyses without control patients, with multiple confounders, eg early v late Rx
The improvement of survival with a high antibody level was a post-hoc determination from stored samples
https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.full.pdf
These">https://www.medrxiv.org/content/1... were 2 exploratory analyses without control patients, with multiple confounders, eg early v late Rx
The improvement of survival with a high antibody level was a post-hoc determination from stored samples
The new FDA website headline "Another Achievement in Administration& #39;s Fight Against Pandemic"
https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment">https://www.fda.gov/news-even... (I have never seen such a statement with any FDA approval)
https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment">https://www.fda.gov/news-even... (I have never seen such a statement with any FDA approval)

 Read on Twitter
Read on Twitter ; there are many trials but they are in progress https://twitter.com/EricTopol... href=" https://twitter.com/EricTopol/status/1296935589728538624">https://twitter.com/EricTopol..." title="3. Type 1 interferon (intranasal) early, preventive therapy has considerable promise (reviewed w/ @VirusesImmunity and thishttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">; there are many trials but they are in progress https://twitter.com/EricTopol... href=" https://twitter.com/EricTopol/status/1296935589728538624">https://twitter.com/EricTopol..." class="img-responsive" style="max-width:100%;"/>
; there are many trials but they are in progress https://twitter.com/EricTopol... href=" https://twitter.com/EricTopol/status/1296935589728538624">https://twitter.com/EricTopol..." title="3. Type 1 interferon (intranasal) early, preventive therapy has considerable promise (reviewed w/ @VirusesImmunity and thishttps://abs.twimg.com/emoji/v2/... draggable="false" alt="🧵" title="Thread" aria-label="Emoji: Thread">; there are many trials but they are in progress https://twitter.com/EricTopol... href=" https://twitter.com/EricTopol/status/1296935589728538624">https://twitter.com/EricTopol..." class="img-responsive" style="max-width:100%;"/>