https://twitter.com/das_seed/status/1291702877384212480">https://twitter.com/das_seed/...
https://twitter.com/das_seed/status/1282627953130704898">https://twitter.com/das_seed/...
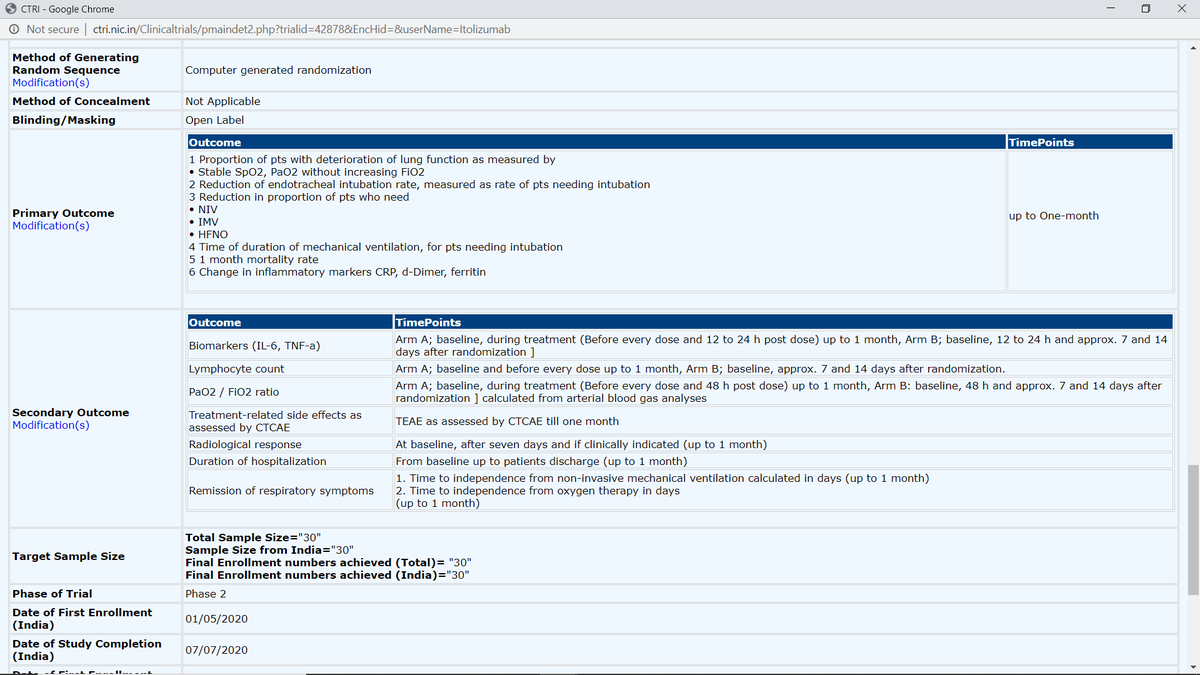
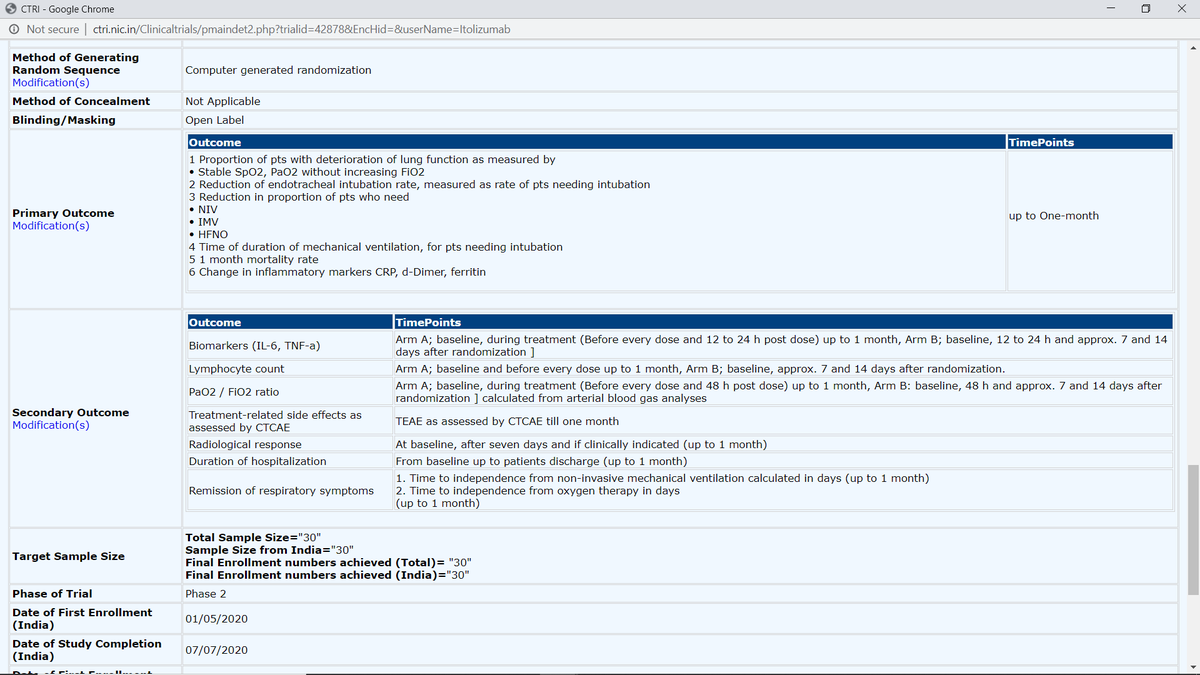
1. Proportion of pts w/ deterioration of lung function as measured by • Stable SpO2, PaO2 w/o increasing FiO2 (was secondary outcome)
2. Reduction of endotracheal intubation rate, measured as rate of pts needing intubation (NEW add)\3
3. Reduction in proportion of pts who need • NIV • IMV •HFNO (NEW add)
4. Time of duration of mechanical ventilation, for pts needing intubation (NEW add)
5. 1-month mortality rate (other than new additions, is initial one LOWERED in preference?) \4 https://twitter.com/das_seed/status/1291702861118799874">https://twitter.com/das_seed/...
4. Time of duration of mechanical ventilation, for pts needing intubation (NEW add)
5. 1-month mortality rate (other than new additions, is initial one LOWERED in preference?) \4 https://twitter.com/das_seed/status/1291702861118799874">https://twitter.com/das_seed/...
6. Change in inflammatory markers CRP (was in secondary outcome), d-Dimer (NEW add), ferritin (NEW add).
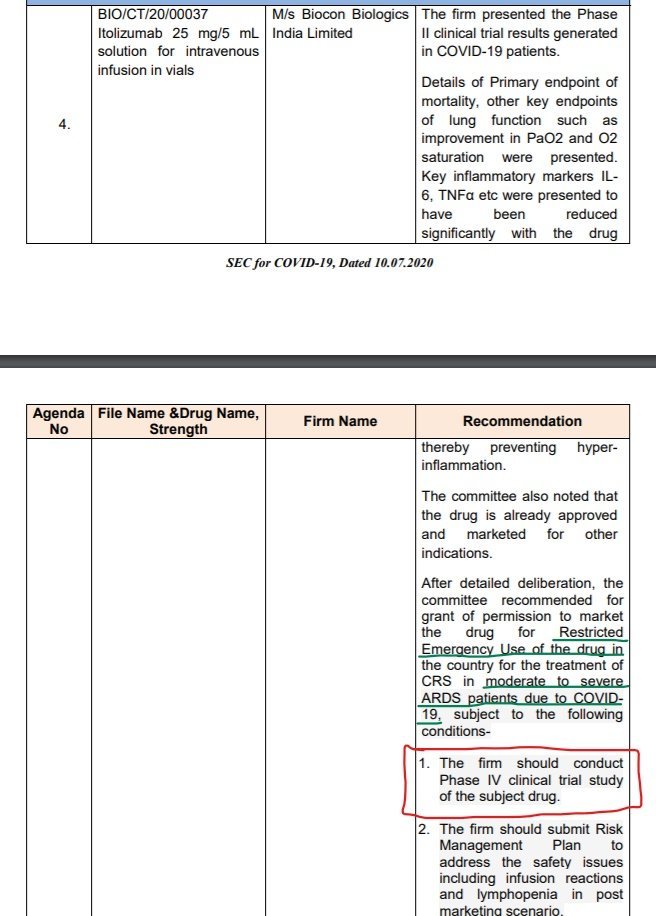
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht">These many additions/modifications to primary outcomes after Restricted Emergency Use & Phase III trial waiver. Are these not RETROSPECTIVE? Is it valid for any RCT? \5
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😱" title="Vor Angst schreiendes Gesicht" aria-label="Emoji: Vor Angst schreiendes Gesicht">These many additions/modifications to primary outcomes after Restricted Emergency Use & Phase III trial waiver. Are these not RETROSPECTIVE? Is it valid for any RCT? \5
Quantitative test mentioned for Biomarkers (IL-6, TNF-a), Lymphocyte count, PaO2/FiO2 ratio, Treatment-related side effects as assessed by CTCAE. \6 https://twitter.com/das_seed/status/1284371948093480960">https://twitter.com/das_seed/...
BUT other critical outcomes left ambiguous in terms of measurement/test: Radiological response, Duration of hospitalization, Remission of respiratory symptoms.
Concerning as post-COVID19 symptoms or affects may appear w/ some delay. What about complications on NIV patients? \7
Concerning as post-COVID19 symptoms or affects may appear w/ some delay. What about complications on NIV patients? \7
Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8
https://twitter.com/das_seed/status/1289917985096835072">https://twitter.com/das_seed/...
Brief summary here:
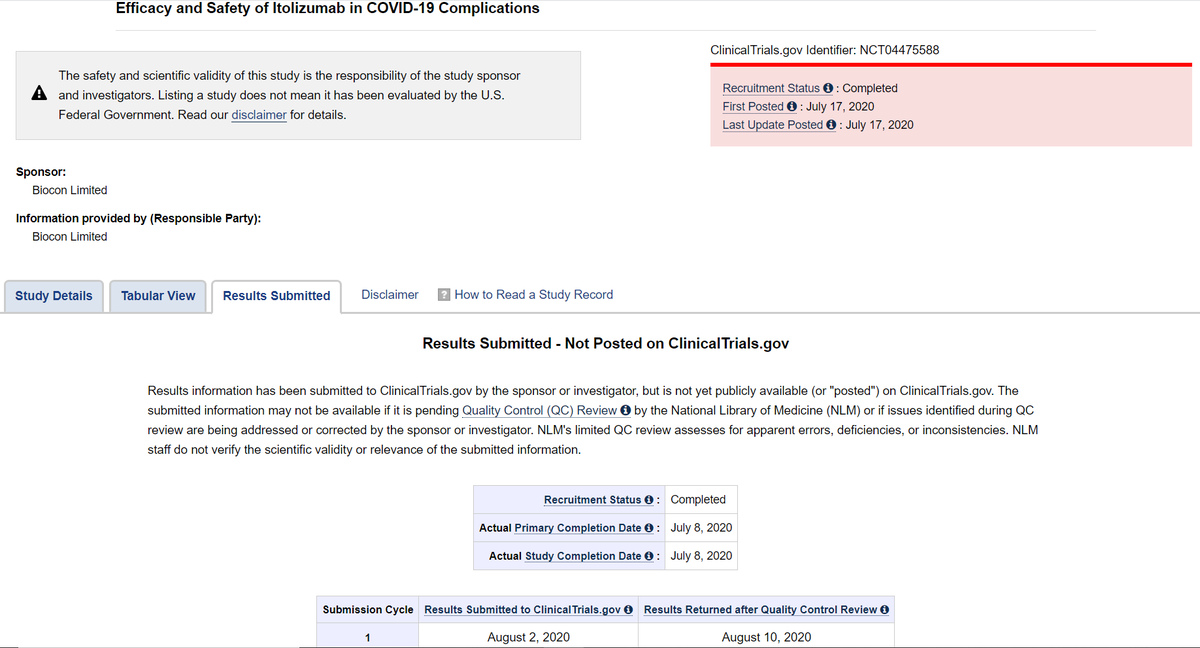
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov"> http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov"> http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020.
On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.
On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.
Outstanding revisions include following.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Primary outcomes.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Primary outcomes.
Initially, 1-month mortality rate was sole primary outcome as approved by SEC/DCGI. Based on this outcome, results of Phase II trial was judged by SEC/DCGI, after which EUA and Phase III trial waiver was granted.
Initially, 1-month mortality rate was sole primary outcome as approved by SEC/DCGI. Based on this outcome, results of Phase II trial was judged by SEC/DCGI, after which EUA and Phase III trial waiver was granted.
However, multiple additions have been done to primary outcomes. Revised primary outcomes include:
1. Proportion of pts w/ deterioration of lung function as measured by • Stable SpO2, PaO2 w/o increasing FiO2
2. Reduction of endotracheal intubation rate, measured as rate of
1. Proportion of pts w/ deterioration of lung function as measured by • Stable SpO2, PaO2 w/o increasing FiO2
2. Reduction of endotracheal intubation rate, measured as rate of
pts needing intubation
3. Reduction in proportion of pts who need • NIV • IMV •HFNO
4. Time of duration of mechanical ventilation, for pts needing intubation
5. 1-month mortality rate
While 1 initially was in secondary outcome, points 2, 3, 4 are new additions to primary.
3. Reduction in proportion of pts who need • NIV • IMV •HFNO
4. Time of duration of mechanical ventilation, for pts needing intubation
5. 1-month mortality rate
While 1 initially was in secondary outcome, points 2, 3, 4 are new additions to primary.
Biomarkers (IL-6, TNF-a), Lymphocyte count, PaO2/FiO2 ratio, Treatment-related side effects as assessed by CTCAE are standard, while
Radiological response, Duration of hospitalization, Remission of respiratory symptoms remain ambiguous.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Dr Shashank Joshi, a member of MH Govt COVID task force got replaced as PI/Coordinator of multicentric trial by Dr Milind Nadkar, KEM.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Dr Shashank Joshi, a member of MH Govt COVID task force got replaced as PI/Coordinator of multicentric trial by Dr Milind Nadkar, KEM.
Completion of trial: 07/07/20
Completion of trial: 07/07/20
Random sequence generator method also added, which is must since trial is supposed to be RCT, and now mentions "Computer generated randomization" (previously, it said "not applicable").
# of sites show 4 (LNJP, Nair H, KEM, AIIMS Delhi).
(pointers https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">) https://twitter.com/das_seed/status/1296047332493074432">https://twitter.com/das_seed/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">) https://twitter.com/das_seed/status/1296047332493074432">https://twitter.com/das_seed/...
# of sites show 4 (LNJP, Nair H, KEM, AIIMS Delhi).
(pointers
1st enrollment was on May 01, 2020 as per CRTI. Nair H had first enrollment on May 18, 2020. KEM had enrolled
https://twitter.com/das_seed/status/1296047346908893185">https://twitter.com/das_seed/...
most patients prior to May 28 as per quotation by KEM officials to @HindustanTimes @RupsaChak where "clinical trial" (30 day for a case) at KEM was over by June 24 itself. LNJP would also have many enrollments done by May 28 as Dr Suresh Kumar mentioned treatment of hospital
staffs infected w/ COVID19 using #Itolizumab after completion of study (probably in his hospital).
We can infer had SIGNIFICANT number of allocations of patients to treatment and control arms were done already by May 28 when SEC noticed issue with randomization process.
We can infer had SIGNIFICANT number of allocations of patients to treatment and control arms were done already by May 28 when SEC noticed issue with randomization process.
This makes an already biased RCT (since, there& #39;s no blinding) to be more biased. Let us note that CTRI regd was on May 01, 2020 and even when CTRI was updated on June, randomization method was stated "not applicable".
It clearly shows Phase II trial is too biased for RCT.
It clearly shows Phase II trial is too biased for RCT.
Site A: 8+4
Site B: 6+3
Site C: 4+2
Site D: 2+1
This distribution is inferred solely from official (BIOCON endorsed) statements & protocol.
Nair H final listing had 9 patients of which 6 were sent to treatment arm & 3 were sent to control arm. LNJP had 8 patients in treatment arm.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/status/1293679743833903104">https://twitter.com/das_seed/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/status/1293679743833903104">https://twitter.com/das_seed/...
Only favorable possibility for @BioconBiologics is that each site maintain 2:1 treatment to control ratio. Any deviation from this is PROOF of rigging the trial MASSIVELY.
Now, we consider another aspect of trial enrollment that raises question over screening process.
Now, we consider another aspect of trial enrollment that raises question over screening process.
3 comorbidities were tallied: Diabities, Hypertension, Hypothyroidism. Out of 30, only 9 patients w/ comorbidities. Patients w/ comorbidities are said to be more vulnerable than those w/o comorbidities. Still ONLY 30% of enrolled patients had comorbidities. Sample size is so
small that only 3 comorbidities were tallied. Extent of severity of these comorbidities are also not clear. And also exclusion criteria that lets PI decide after review of patients condition makes selection process ambiguous for RCT.
@pash22 @prat1112001
https://twitter.com/das_seed/status/1291011602226065408">https://twitter.com/das_seed/...
@pash22 @prat1112001
https://twitter.com/das_seed/status/1291011602226065408">https://twitter.com/das_seed/...

 Read on Twitter
Read on Twitter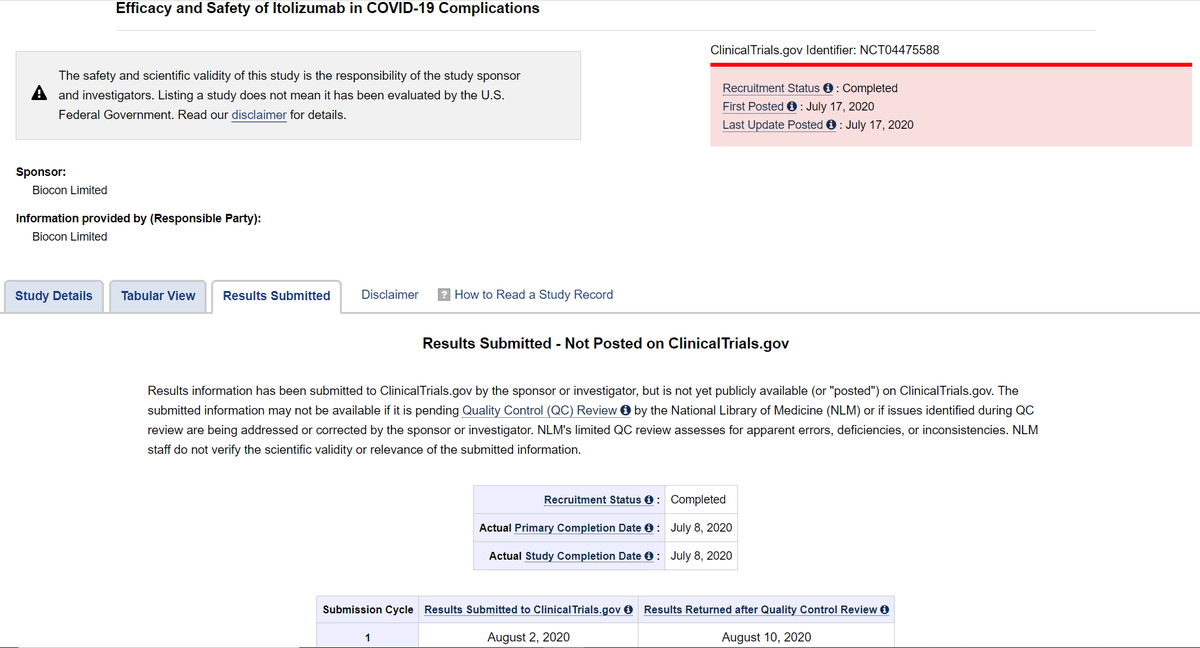 @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐒" title="Affe" aria-label="Emoji: Affe"> @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/...">
@BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐒" title="Affe" aria-label="Emoji: Affe"> @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/...">
 @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐒" title="Affe" aria-label="Emoji: Affe"> @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/...">
@BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐒" title="Affe" aria-label="Emoji: Affe"> @BioconBiologics modified CTRI/2020/05/024959 #Itolizumab #COVID19 on Aug 20, 2020 (> 40 days of trial completion). (https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧐" title="Gesicht mit Monokel" aria-label="Emoji: Gesicht mit Monokel"> this thread) https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Results Submitted to http://ClinicalTrials.gov on Aug 2 and Results Returned after Quality Control Review on Aug 10. \1 https://twitter.com/das_seed/...">
 Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥷" title="Ninja" aria-label="Emoji: Ninja"> Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/...">
Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥷" title="Ninja" aria-label="Emoji: Ninja"> Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/...">
 Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥷" title="Ninja" aria-label="Emoji: Ninja"> Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/...">
Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="🥷" title="Ninja" aria-label="Emoji: Ninja"> Primary Outcomes revised. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">A/c to SEC/DCGI (initial): 1-month mortality rate b/n two arms, time frame: 1-month. 1-month mortality is defined as the ratio of patients who will live after 1 month from study start out of those registered at baseline.\2 https://twitter.com/das_seed/...">
 Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/...">
Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/...">
 Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/...">
Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Dr Shashank Joshi is replaced by Dr Milind Nadkar, KEM as PI or Trial Coordinator of multicentric study. Dr Shashank Joshi is also member of MH Govt COVID19 Task Force, once also agreed on TV that large RCT trial is needed for #Itolizumab #COVID19. \8 https://twitter.com/das_seed/...">
 Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
 Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
 Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
 Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details." title="Brief summary here: https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts"> Biocon Biologics had submitted result to http://ClinicalTrials.gov @NIH on Aug 02, 2020 BUT results were returned after quality control review on Aug 10, 2020. On Aug 18, 2020 Biocon Biologics made modifications to Indian CTRI registration details.">
 Serious systematic bias in already open-label trial a/c to statements from SEC and BIOCON.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SEC noted that "Randomization wasn& #39;t proper".1st enrollment was on May 01, 2020 as per CRTI. Nair H had first enrollment on May 18, 2020. KEM had enrolled https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Serious systematic bias in already open-label trial a/c to statements from SEC and BIOCON.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SEC noted that "Randomization wasn& #39;t proper".1st enrollment was on May 01, 2020 as per CRTI. Nair H had first enrollment on May 18, 2020. KEM had enrolled https://twitter.com/das_seed/..." class="img-responsive" style="max-width:100%;"/>
Serious systematic bias in already open-label trial a/c to statements from SEC and BIOCON.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SEC noted that "Randomization wasn& #39;t proper".1st enrollment was on May 01, 2020 as per CRTI. Nair H had first enrollment on May 18, 2020. KEM had enrolled https://twitter.com/das_seed/..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="👉" title="Rückhand Zeigefinger nach rechts" aria-label="Emoji: Rückhand Zeigefinger nach rechts">Serious systematic bias in already open-label trial a/c to statements from SEC and BIOCON.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SEC noted that "Randomization wasn& #39;t proper".1st enrollment was on May 01, 2020 as per CRTI. Nair H had first enrollment on May 18, 2020. KEM had enrolled https://twitter.com/das_seed/..." class="img-responsive" style="max-width:100%;"/>
 This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/..." title="Nair H final listing had 9 patients of which 6 were sent to treatment arm & 3 were sent to control arm. LNJP had 8 patients in treatment arm. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/..." class="img-responsive" style="max-width:100%;"/>
This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/..." title="Nair H final listing had 9 patients of which 6 were sent to treatment arm & 3 were sent to control arm. LNJP had 8 patients in treatment arm. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">This RULES OUT centralized randomization & allocation of patients to treatment & control at given sites. https://twitter.com/das_seed/..." class="img-responsive" style="max-width:100%;"/>