might fuck around and do a long detailed t-cell thread
there& #39;s a lovely new paper that sheds a lot of light on CD8+ (aka killer/cytotoxic) T cells in COVID-19 and helps address some of the recent muddle about cross-immunity from the endemic/"common cold" coronaviruses
https://www.biorxiv.org/content/10.1101/2020.08.13.249433v1.full.pdf">https://www.biorxiv.org/content/1...
https://www.biorxiv.org/content/10.1101/2020.08.13.249433v1.full.pdf">https://www.biorxiv.org/content/1...
despite my well-known fondness for t cells, i& #39;ve been skeptical that t cells elicited by prior coronavirus infections are playing a major role in COVID disease severity (positive or negative). the basic reason is that SARS2 is not very genetically similar to those viruses.
the way t cells work is by recognizing specific "epitopes": peptides (short chains of amino acids) derived from pathogenic proteins. yes, there are some bits of SARS2 that are similar to bits within other coronaviruses, but not a ton.
and what are the odds that, when you got infected with, say, OC43 (another coronavirus), your t cells specifically targeted one of the little bits that happens to be similar to a little bit within SARS2? it would just be dumb luck
moreover, how much of a leg up would that give you? plausibly not a huge one. at the time of infection, you& #39;d have some more t cells available that would already be able to attack virus-infected cells. but how many more? beneficial at the margin, maybe, but prob not huge
another important background point: t cells recognize little peptides when they presented on the surface of infected cells via special proteins. there are many different variations on these proteins across people and ethnic groups. this affects which peptides are targeted too
these different variations on the genes (alleles) are called things like HLA-A*02 and HLA-B*44 etc. what this new study does is use existing algorithms to predict, for a given HLA allele, what peptides derived from SARS2 are most likely to get recognized by a person& #39;s T cells
the researchers then take a couple dozen people who have recovered from mild cases of COVID and look into their blood to see if there are any t cells in there that match what the algorithms predict. and yes! they find such killer t cells in almost every case.
now, what if you look at people who have NEVER been infected, using blood samples from last year? do they too have cells (at a high enough frequency to detect) that recognize these validated epitopes? they answer here is generally...no
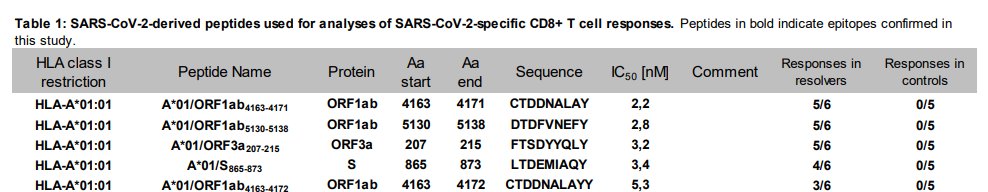
to make it a little more concrete, let& #39;s focus on a single genetic subgroup, people with HLA-A*01:01. this is like 15-20% of Europeans and white Americans but rare in African Americans or in Shanghai (see Extended Data Fig. 1)
the researchers predicted the five best epitopes from SARS2 for such people to recognize. and sure enough, these are epitopes that most such people respond to
"sequence" shows the set of amino acids in the peptide (each letter stands for an amino acid). "responses in resolvers" tells you how many of the COVID patients in that genetic group test positive for T cells that bind to the amino-acid sequence in that row.
so solid majorities of the (few) patients tested did in fact respond to those peptides/epitopes. but look at the final column. in every case, t cells responding to these epitopes could not be detected in historical samples from pre-COVID times
so in this genetic group, the epitopes that most people respond to probably having nothing to do with the common cold coronaviruses. same is true for almost all the other HLA groups they have data for
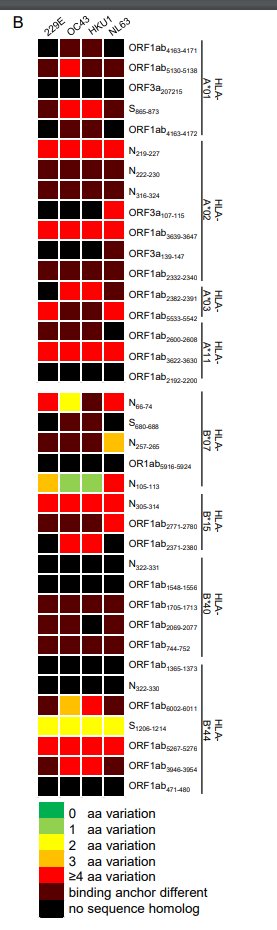
this is made even clearer in this little chart. each row is an epitope/peptide/amino-acid sequence that at least one of these COVID patients responds to in a significant way. they are grouped by HLA subset.
black/red means these bits of the SARS2 virus are not similar to corresponding bits from other coronaviruses. green means they are similar. what you see is...a lot of black and red. the major epitopes are bits of SARS2 that are not really shared by its cousins
HOWEVER! there is one interesting exception! a particular peptide, running from amino acid 105 to 113 of the nucleoprotein, is very similar to the same bit of the nucleoproteins from the coronaviruses OC43 and HKU1. for SARS, it& #39;s SPRWYFYYL. for OC43, it& #39;s LPRWYFYYL. just one Δ
this peptide is predicted to be a good fit for the HLA-B*07 group. sure enough, most COVID patients from that group have t cells that recognize it (6 out of 7). but so 2 out of 5 of the uninfected historical samples!
the researchers grab another set of 6 uninfected people in this HLA group. 3 of them have t cells that recognize this SARS2 peptide! so yes, this is probably the holy grail: a common coronavirus epitope that is also a SARS2 epitope
but wait a second...is this really a big deal? so in maybe half of all people in this particular genetic group, which is itself not a huge % of all people in the world (maybe 10% in the US?), there is an elevated number of killer t cells at baseline that could respond to SARS2
and...there aren& #39;t that many of these cells even in the people who test positive for them. it& #39;s around 1 in 250,000 killer t cells in their blood
is that better than nothing? yeah, i would guess so. is it likely to make a huge difference? i would guess not. after SARS2 infection this only gets boosted to around 1 in 100,000. there are almost many other epitopes that come into play. it& #39;s not a silver bullet
this is the huge problem w/the studies i& #39;ve seen so far that say that unexposed people have SARS2-responsive t cells. i& #39;m sure they do. you have t cells for everything under the sun! the problem is that each kind starts out very very rare
if you do tricks to amplify cells from random people, you& #39;ll find some that responds to anything. but if there are very few of these cells inside a person under normal conditions, it& #39;s still going to take a while for the immune system to find them, get them to multiply, etc.
caveat: other epitopes will be found, and there may be more that are similar to epitopes derived from other coronaviruses. but at the moment, i& #39;m not seeing the evidence that these shared epitopes are playing a major role in the typical immune response to COVID.
oh yeah, i also went through a lot of similar points a little while ago with respect to a paper on CD4+ helper (as opposed to CD8+ killer) t cells so check that out if you wanna. i& #39;ll stop now https://twitter.com/LucreSnooker/status/1291228210894639105">https://twitter.com/LucreSnoo...
worth pointing out that another paper from a different group using different methods, which generally also finds "minimal cross-reactivity of SARS-CoV-2-reactive memory CD8+ T cells with other coronaviruses," identified one notable exception, and it& #39;s the exact same one!
the paper is https://www.medrxiv.org/content/10.1101/2020.07.24.20161653v1.full.pdf">https://www.medrxiv.org/content/1... and the exception is the peptide SPRWYFYYL. but again, this only seems to be relevant to the subset of people with the HLA-B*07 allele. still, cool that these findings replicate
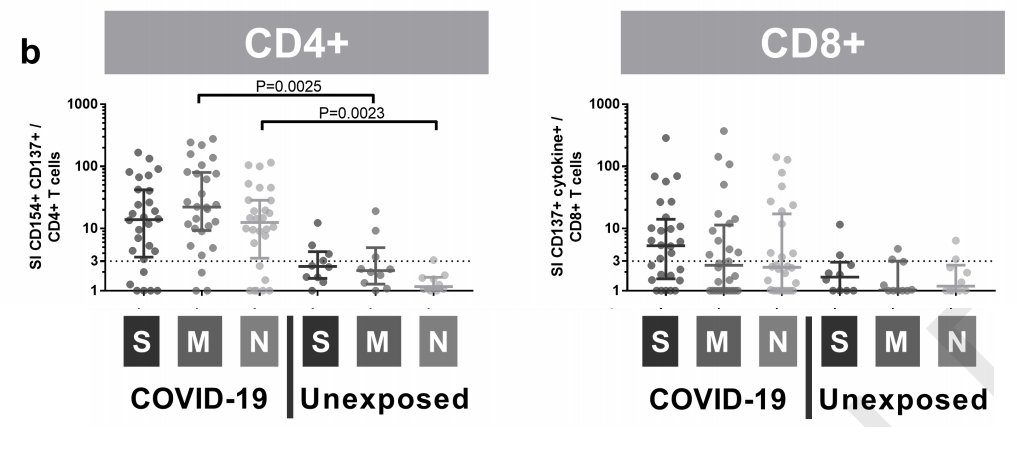
another t-cell paper: if you expose healthy people& #39;s t cells to peptides derived from three SARS2 proteins, some of them kind of respond...maybe...but not much and not in many patients
S, M, and N are three viral proteins. the y-axis values are a "stimulation index" -- basically, when you expose cells to bits of this protein, how many of them show various activation markers relative to cells exposed to nothing. the unexposed cells do often "respond" in this way
...which is why they count a stimulation index below 3 as a non-response. you can see a few blips among unexposed people but it& #39;s not hard to tell the difference between the infected people and the uninfected people
( https://www.cell.com/action/showPdf?pii=S2666-3791%2820%2930118-X)">https://www.cell.com/action/sh...
( https://www.cell.com/action/showPdf?pii=S2666-3791%2820%2930118-X)">https://www.cell.com/action/sh...

 Read on Twitter
Read on Twitter