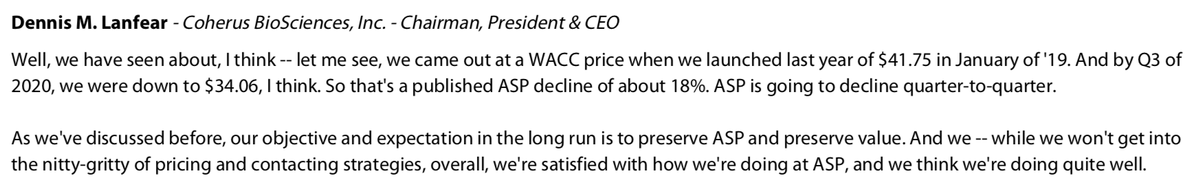Been posting about $CHRS a lot lately but the recent earnings call was very bullish to me so I& #39;m going to post the Q&A answers I was most interested in...(The prepared remarks were good, too, but the Q&A hits on the most common bearish POVs.)
On branded Neulasta& #39;s current share and total pegfilgrastim market growth
On Udenyca& #39;s market share exiting the quarter
On business development. Oncology is still the focus...even if they did no business development they are on track for 5 biosimiliar launches in the next 5 years. That will get the focus off nitpicking minor Udenyca fluctuations
On PD-1 strategy...good chance this is the next focus once they get through the 2025 approvals and launch if not sooner
On Gross Margins...maybe the most interesting answer. This business is a very attractive space to be in
All in all, I think the pegfilgrastim market looks like this currently:
Neulasta OnPro: 56%
Neulasta Legacy PFS product: 14%
Udenyca (Coherus): 23%
Fulphila (Mylan): 7%
Ziextenzo (Sandoz) <1%
Pfizer - awaiting code
Neulasta OnPro: 56%
Neulasta Legacy PFS product: 14%
Udenyca (Coherus): 23%
Fulphila (Mylan): 7%
Ziextenzo (Sandoz) <1%
Pfizer - awaiting code
I think OnPro probable settles around 50%. Leaving half the market for the biosims to fight over and this is a market that is growing with new treatment guidelines...eventually there will be over a million US patients per year using these products.
Pricing is a concern but the biosimiliar space is a lot more than pure price as you have to handhold the providers, patients with copay cards, etc. Coherus has a completely US-based supply chain which may be a small benefit.
But the real focus should be using Udenyca& #39;s profitability to bridge until end of 2021 when Lucentis biosimiliar should launch... (will be watching this to assess management credibility) Avastin biosimiliar should come late 2021/early 2022 as well.
$chrs timeline:
Late 2021: Lucentis biosim
Early 2022: Avastin biosim
2023: Rituxan and Humira biosims
2025: Eylea biosim
Total addressable market is $30b. Even capturing 20% of that while keeping costs low...
Late 2021: Lucentis biosim
Early 2022: Avastin biosim
2023: Rituxan and Humira biosims
2025: Eylea biosim
Total addressable market is $30b. Even capturing 20% of that while keeping costs low...
Humira market will be hard to capture 20% share because of competition but market is so large ($15b) that you don& #39;t need necessarily...
Lucentis market is much smaller ($1.8b) but competition will be limited due to difficulties to produce the product so possibility of more share
Lucentis market is much smaller ($1.8b) but competition will be limited due to difficulties to produce the product so possibility of more share
Also Neulasta might have an on body product but they aren& #39;t commenting due to competitive reasons. I& #39;m not expecting it any time soon but would be very bullish to go after the half of the market Udenyca can& #39;t really compete with.
Threat to the wAMD biosimiliars would be a superior product coming along to treat wAMD so probably good idea to get long the product you think will do that...
End of thread-bearish viewpoints welcome on competition, products they licensed, etc. Short % of float is 30, seems high
End of thread-bearish viewpoints welcome on competition, products they licensed, etc. Short % of float is 30, seems high

 Read on Twitter
Read on Twitter