[QUICK THREAD: CANNON TO KILL VIRUSES?]
1/21
This news attached here made headlines on all mainstream rags earlier this month. It speaks of a groundbreaking device that can kill coronaviruses with electron beams. https://health.economictimes.indiatimes.com/news/medical-devices/bengaluru-organisation-gets-us-fda-eu-nod-for-device-that-can-kill-covid-19-virus/77311333">https://health.economictimes.indiatimes.com/news/medi...
1/21
This news attached here made headlines on all mainstream rags earlier this month. It speaks of a groundbreaking device that can kill coronaviruses with electron beams. https://health.economictimes.indiatimes.com/news/medical-devices/bengaluru-organisation-gets-us-fda-eu-nod-for-device-that-can-kill-covid-19-virus/77311333">https://health.economictimes.indiatimes.com/news/medi...
2/21
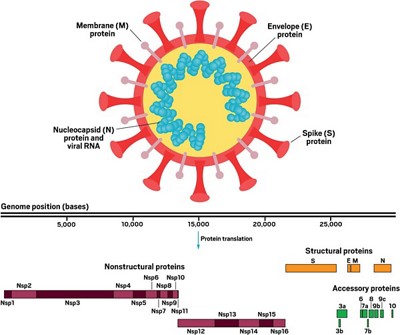
It calls the device SHYCOCAN, short for Scalene Hypercharge Corona Cannon, and claims to run off a regular wall socket. The mechanism involves exciting a bunch of electrons and then beaming them out. These electrons then hit coronaviruses and render then harmless.
It calls the device SHYCOCAN, short for Scalene Hypercharge Corona Cannon, and claims to run off a regular wall socket. The mechanism involves exciting a bunch of electrons and then beaming them out. These electrons then hit coronaviruses and render then harmless.
3/21
This happens because coronaviruses have spikes on their surface called S-protein. These spikes are positively charged and help unlock human cells to get the virus in. Electrons in the beam, should they hit a stray coronavirus, serve to neutralize these spikes.
This happens because coronaviruses have spikes on their surface called S-protein. These spikes are positively charged and help unlock human cells to get the virus in. Electrons in the beam, should they hit a stray coronavirus, serve to neutralize these spikes.
4/21
The science does seem to check out, at least to my layman eyes, although I& #39;d defer further examination to an expert ( @Neurophysik?). Here& #39;s a paper by two molecular biologists from the University of Western Cape, Cape Town, that seems to corroborate. https://virologyj.biomedcentral.com/articles/10.1186/s12985-019-1182-0">https://virologyj.biomedcentral.com/articles/...
The science does seem to check out, at least to my layman eyes, although I& #39;d defer further examination to an expert ( @Neurophysik?). Here& #39;s a paper by two molecular biologists from the University of Western Cape, Cape Town, that seems to corroborate. https://virologyj.biomedcentral.com/articles/10.1186/s12985-019-1182-0">https://virologyj.biomedcentral.com/articles/...
5/21
One question still remains. Does the claimed device actually exist and, if so, does it have the approval being claimed? The @EconomicTimes article here (there& #39;s others in @DeccanHerald, @timesofindia, etc.) claims both @US_FDA and EU approvals. Great!
One question still remains. Does the claimed device actually exist and, if so, does it have the approval being claimed? The @EconomicTimes article here (there& #39;s others in @DeccanHerald, @timesofindia, etc.) claims both @US_FDA and EU approvals. Great!
6/21
Only way to determine, I shot an inquiry to the FDA. That was Aug 2. Minutes ago, I received a response from their Center for Devices and Radiological Health or CDRH. You may read it here:
Only way to determine, I shot an inquiry to the FDA. That was Aug 2. Minutes ago, I received a response from their Center for Devices and Radiological Health or CDRH. You may read it here:
7/21
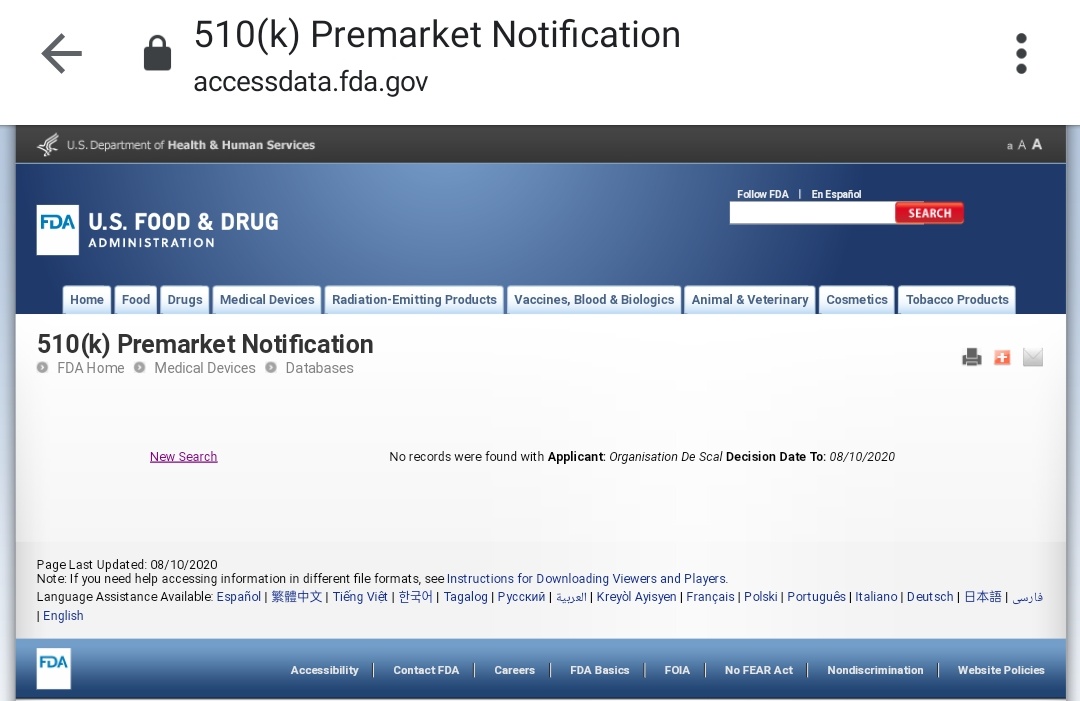
Per this response, they have 3 databases where any such device either approved or pending approval could be looked up. The first such database is titled "510(k) Premarket Notification" (PMN). Here& #39;s a link to the query:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm">https://www.accessdata.fda.gov/scripts/c...
Per this response, they have 3 databases where any such device either approved or pending approval could be looked up. The first such database is titled "510(k) Premarket Notification" (PMN). Here& #39;s a link to the query:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm">https://www.accessdata.fda.gov/scripts/c...
8/21
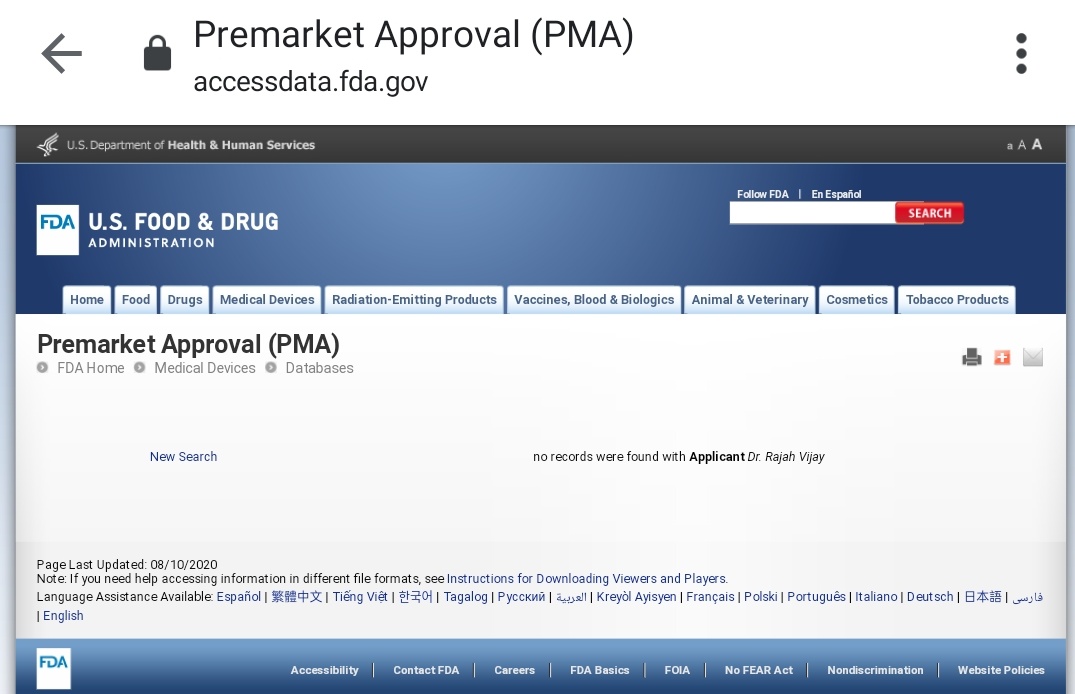
The second is titled "Premarket Approval" (PMA) and a query against it can be accessed at the following link:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm">https://www.accessdata.fda.gov/scripts/c...
The second is titled "Premarket Approval" (PMA) and a query against it can be accessed at the following link:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm">https://www.accessdata.fda.gov/scripts/c...
9/21
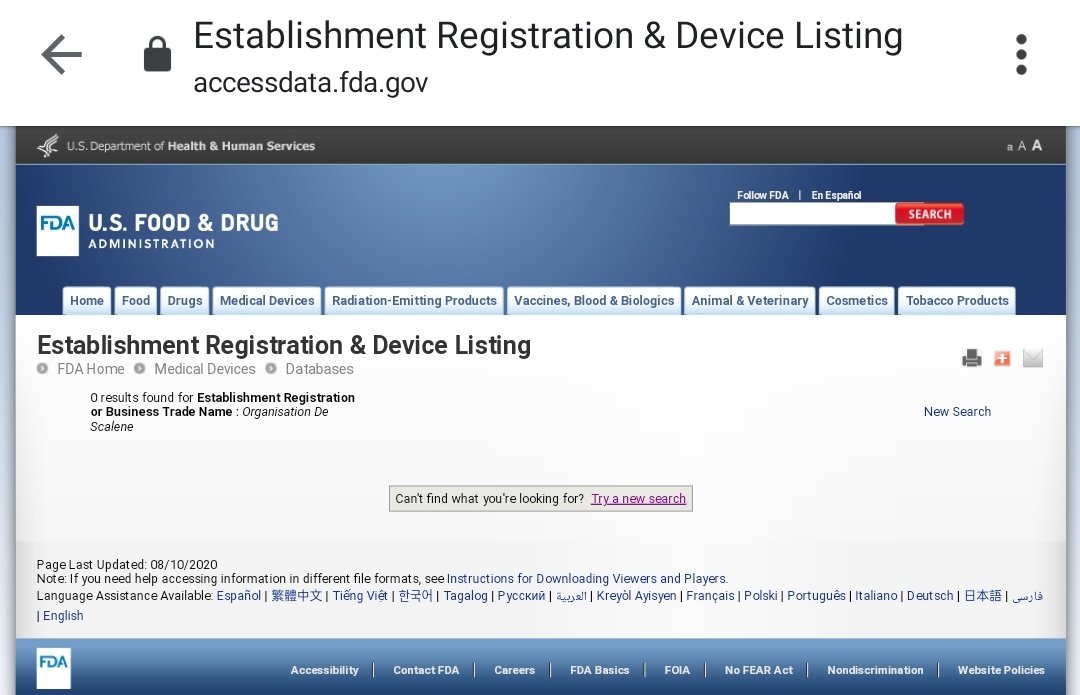
The third database offered is titled "Establishment Registration & Device Listing" (ERDL). This database can be looked up using the query available at:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm">https://www.accessdata.fda.gov/scripts/c...
The third database offered is titled "Establishment Registration & Device Listing" (ERDL). This database can be looked up using the query available at:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm">https://www.accessdata.fda.gov/scripts/c...
10/21
Of these, PMN and PMA carry information on devices and their approval status. These are updated once a week and up to a month for new clearances to be added. Lookup may be performed using company name, device name, and any of several other variables.
Of these, PMN and PMA carry information on devices and their approval status. These are updated once a week and up to a month for new clearances to be added. Lookup may be performed using company name, device name, and any of several other variables.
11/21
The ERDL database offers information not on devices but businesses. If a business is registered with the FDA for any approval, it should show up here.
So these are the 3 databases of concern to us, two for products and one for their makers.
The ERDL database offers information not on devices but businesses. If a business is registered with the FDA for any approval, it should show up here.
So these are the 3 databases of concern to us, two for products and one for their makers.
12/21
I tried looking up the products first. For some reason, all searches returned negative. Just to recall, the device is named SHYCOCAN, short for Scalene Hypercharge Corona Cannon. Here& #39;s the results off PMN:
I tried looking up the products first. For some reason, all searches returned negative. Just to recall, the device is named SHYCOCAN, short for Scalene Hypercharge Corona Cannon. Here& #39;s the results off PMN:
14/21
Do note that both PMN and PMA may take up to a month to show any new clearance as per FDA& #39;s own admission, so it& #39;s technically possible that the nod has been acquired as claimed but the update is pending. Benefit of doubt? Sure, except...
Do note that both PMN and PMA may take up to a month to show any new clearance as per FDA& #39;s own admission, so it& #39;s technically possible that the nod has been acquired as claimed but the update is pending. Benefit of doubt? Sure, except...
15/21
In the preceding tweets, do note 3 names:
- Organisation de Scalene, the company,
- Dr. Rajah Vijay, the Chairman, and
- Bangalore, India, the location.
With this information, I headed for the 3rd database. The one listing establishments, the ERDL.
In the preceding tweets, do note 3 names:
- Organisation de Scalene, the company,
- Dr. Rajah Vijay, the Chairman, and
- Bangalore, India, the location.
With this information, I headed for the 3rd database. The one listing establishments, the ERDL.
16/21
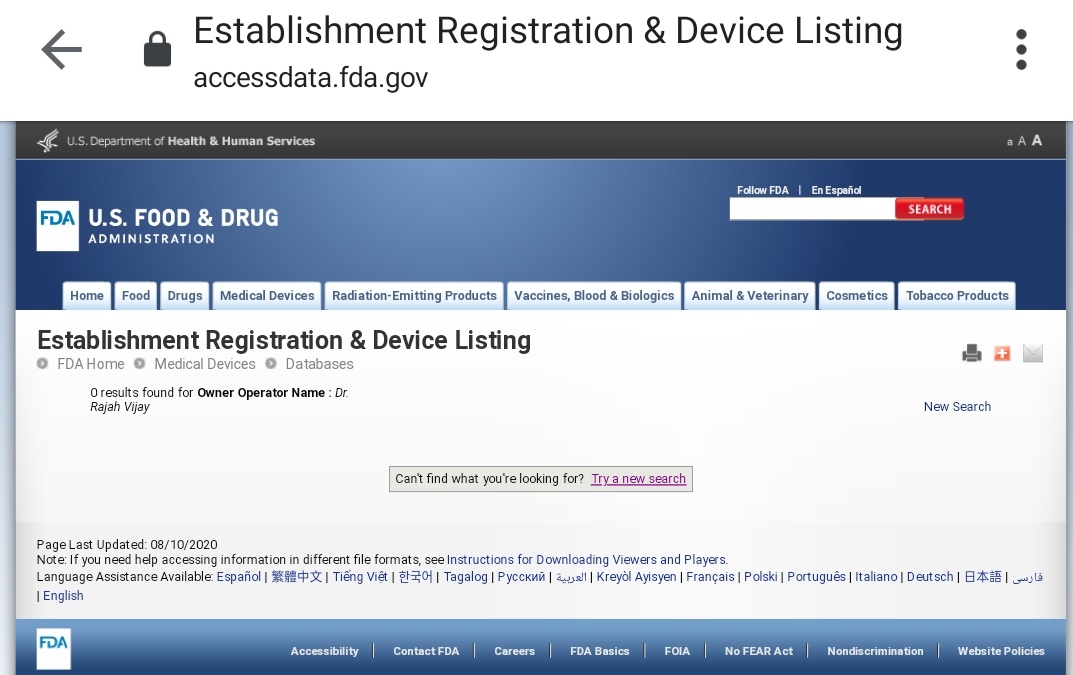
If Organisation de Scalene has ever applied for any FDA approval, my layman understanding is that it should first be registered with it as an applicant in which case, it should show up in ERDL queries. Let& #39;s see:
If Organisation de Scalene has ever applied for any FDA approval, my layman understanding is that it should first be registered with it as an applicant in which case, it should show up in ERDL queries. Let& #39;s see:
17/21
Just to be extra sure, I tried one last lookup, country search. I just selected India in the country drop-down and it threw up a list of all establishments registered from this country. No sign of an Organisation de Scalene, nor any of its parent entity, CARD.
Just to be extra sure, I tried one last lookup, country search. I just selected India in the country drop-down and it threw up a list of all establishments registered from this country. No sign of an Organisation de Scalene, nor any of its parent entity, CARD.
18/21
At this point, it seems narrower the miracle coronavirus zapper nor its maker find any mention anywhere on any FDA listing. But here& #39;s more.
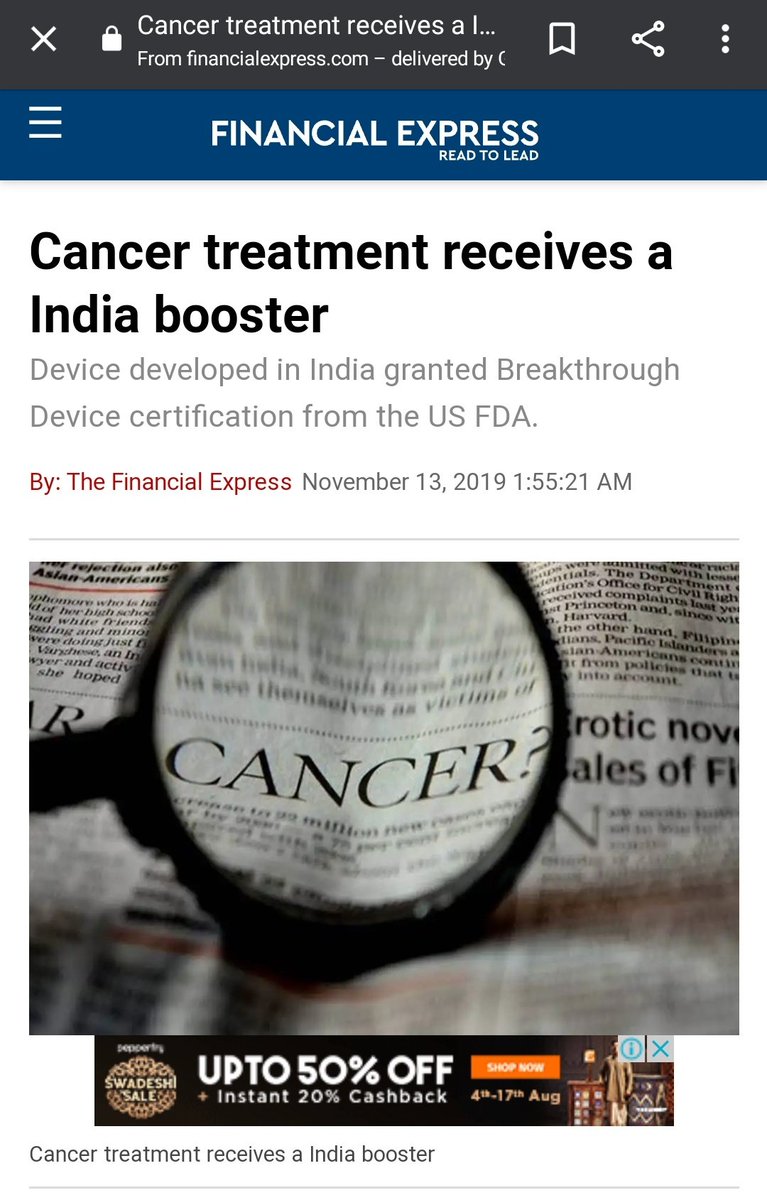
Dr. Rajah Vijay Kumar.
Man& #39;s a prodigy! Here& #39;s a piece from last year claiming another FDA nod.
https://www.financialexpress.com/opinion/cancer-treatment-receives-a-india-booster/1762918/">https://www.financialexpress.com/opinion/c...
At this point, it seems narrower the miracle coronavirus zapper nor its maker find any mention anywhere on any FDA listing. But here& #39;s more.
Dr. Rajah Vijay Kumar.
Man& #39;s a prodigy! Here& #39;s a piece from last year claiming another FDA nod.
https://www.financialexpress.com/opinion/cancer-treatment-receives-a-india-booster/1762918/">https://www.financialexpress.com/opinion/c...
19/21
What that Nov 2019 piece claims is that Dr. Rajah Vijay Kumar has invented a device called "cytotron," a device that uses targeted radio waves to kill cancer tumors. Stop I looked up cytotron in both PMN and PMA. Result? Here:
What that Nov 2019 piece claims is that Dr. Rajah Vijay Kumar has invented a device called "cytotron," a device that uses targeted radio waves to kill cancer tumors. Stop I looked up cytotron in both PMN and PMA. Result? Here:
20/21
The claim is from November last year. That& #39;s 9 whole months, long enough for the slowest red tape at the FDA to have concluded the process. I thought cytotron was imaginary.
Turns out, someone else did too. A good 10 years earlier. http://nirmukta.com/2010/05/06/cancer-cure-scam-cytotron-therapy-ad-continues-to-run-in-newspapers/">https://nirmukta.com/2010/05/0...
The claim is from November last year. That& #39;s 9 whole months, long enough for the slowest red tape at the FDA to have concluded the process. I thought cytotron was imaginary.
Turns out, someone else did too. A good 10 years earlier. http://nirmukta.com/2010/05/06/cancer-cure-scam-cytotron-therapy-ad-continues-to-run-in-newspapers/">https://nirmukta.com/2010/05/0...
21/21
At this point, you could do one of the 2 things. Either give them the benefit of the doubt that the coronavirus cannon actually exists and is only awaiting database updation, or go by available evidences against all such claims, both past and present.
Choice is yours.
At this point, you could do one of the 2 things. Either give them the benefit of the doubt that the coronavirus cannon actually exists and is only awaiting database updation, or go by available evidences against all such claims, both past and present.
Choice is yours.

 Read on Twitter
Read on Twitter