Taking the excellent article by Suzanne Haber, @yendiki
and @SaadJbabdi in BioPsych as an opportunity for a thread:
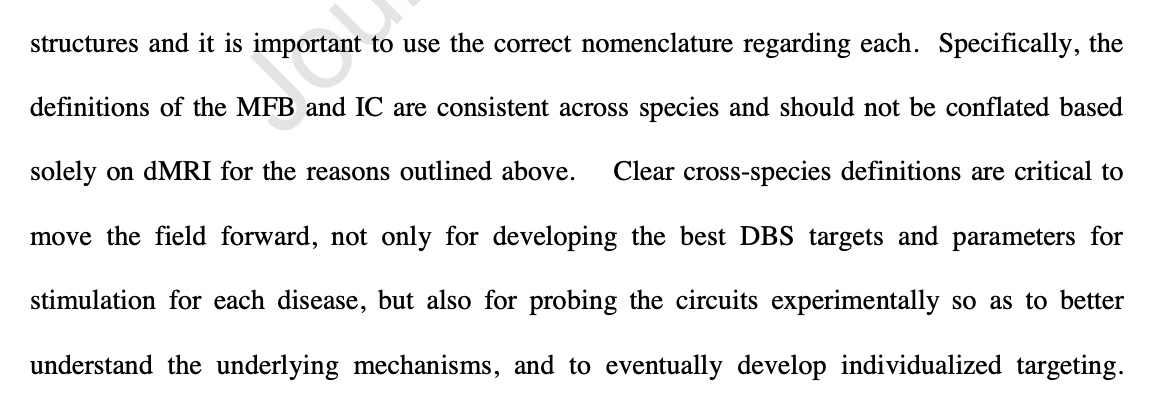
“The dMRI tractography streamlines labeled as the slMFB represent fibers of the Internal Capsule, not the MFB.”
I could not agree more.
https://www.biologicalpsychiatryjournal.com/article/S0006-3223(20)31773-X/fulltext">https://www.biologicalpsychiatryjournal.com/article/S...
and @SaadJbabdi in BioPsych as an opportunity for a thread:
“The dMRI tractography streamlines labeled as the slMFB represent fibers of the Internal Capsule, not the MFB.”
I could not agree more.
https://www.biologicalpsychiatryjournal.com/article/S0006-3223(20)31773-X/fulltext">https://www.biologicalpsychiatryjournal.com/article/S...
The original idea of the slMFB target comes from Coenen et al in 2009 – single PD patient had transient, stimulation- induced hypomanic episode & showed conn between 1 active contact via limbic STN “tributaries” to the MFB. These were identified by DTI.
http://doi.org/10.1227/01.NEU.0000345631.54446.06">https://doi.org/10.1227/0...
http://doi.org/10.1227/01.NEU.0000345631.54446.06">https://doi.org/10.1227/0...
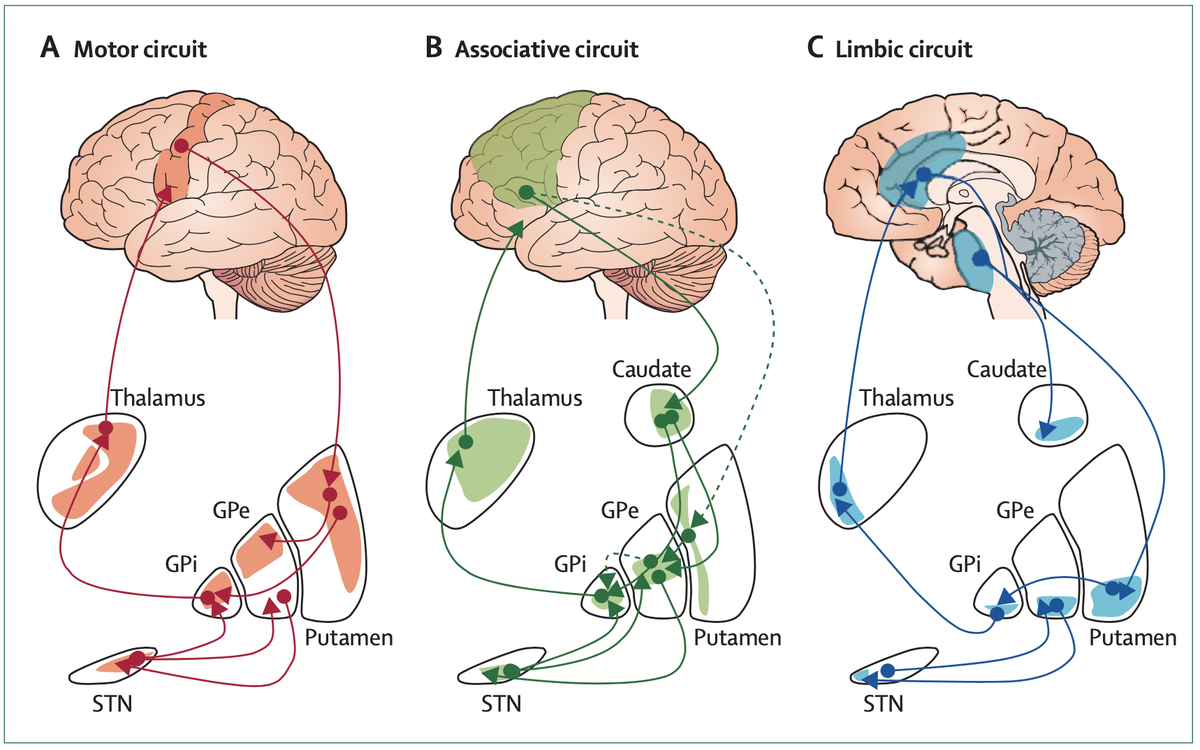
To interpret the occurence of hypomania in STN-DBS based on MFB-stim was surprising, since there already was an accepted model of how this would happen: STN is connected to the whole frontal cortex, different loops lead to modulation of different symptoms. http://doi.org/10.1016/S1474-4422(09)70293-5">https://doi.org/10.1016/S...
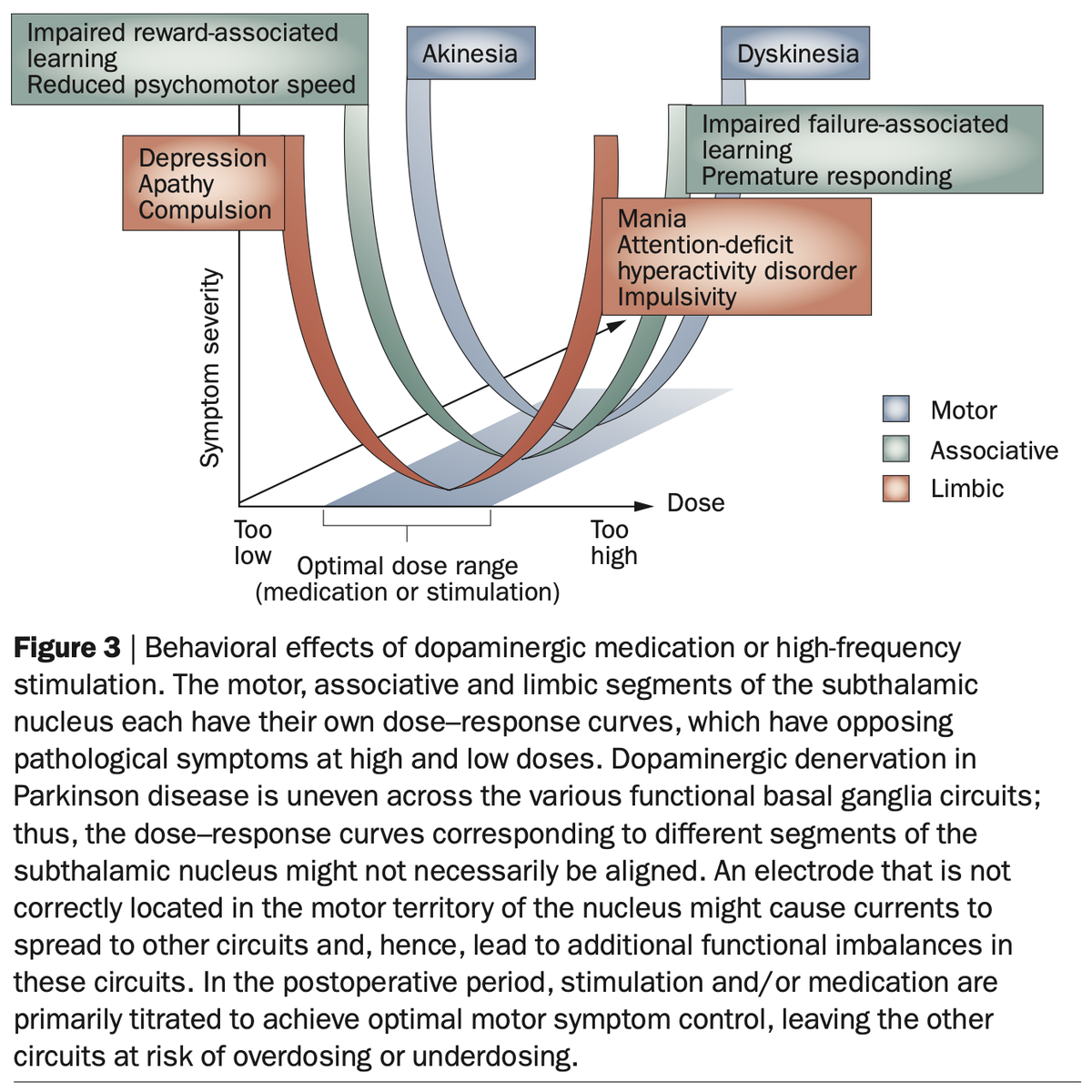
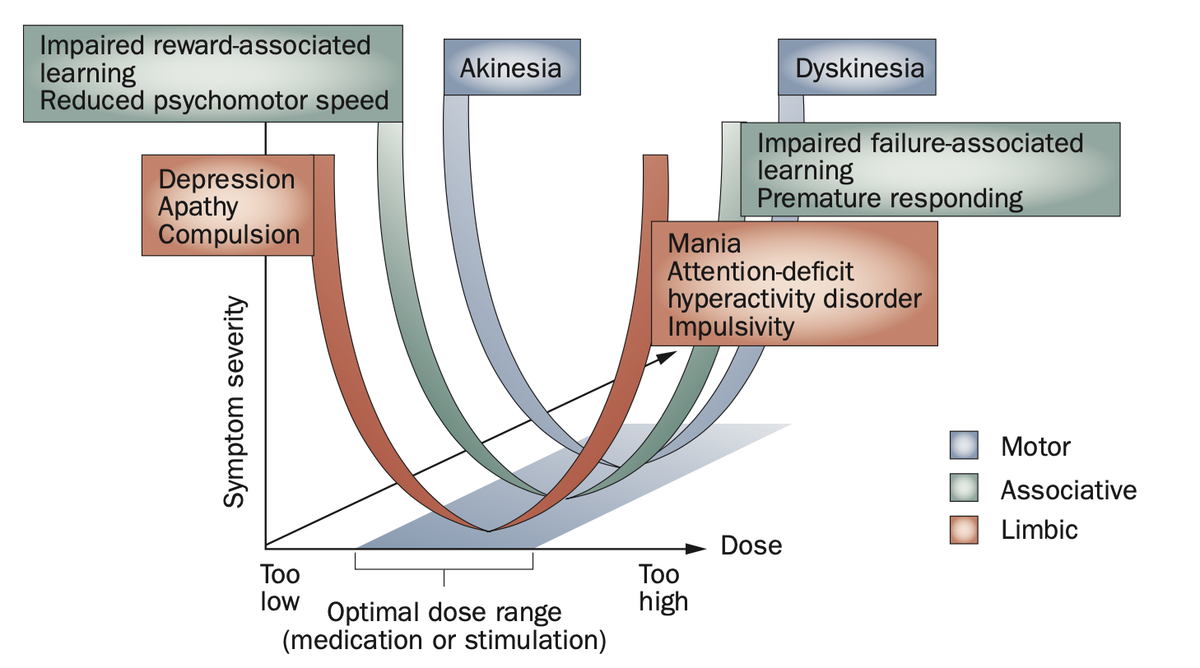
A review by Volkmann and colleagues in @NatRevNeurol expands on this by contrasting motor symptoms in PD to nonmotor symptoms involved in other basal ganglia disorders. Hence, hypomania as a side-effect of STN-DBS has a good pathophysiological model.
http://doi.org/10.1038/nrneurol.2010.111">https://doi.org/10.1038/n...
http://doi.org/10.1038/nrneurol.2010.111">https://doi.org/10.1038/n...
Still, these small green bundles termed „tributaries“ led to a different conclusion and brought the MFB to modern-day DBS.
(NB: Robert Heath had already stimulated the MFB in the 1950ies, but likely an anatomically correct one, see below).
(NB: Robert Heath had already stimulated the MFB in the 1950ies, but likely an anatomically correct one, see below).
…particularly troublesome since average true-positive fiber-tract is matched by four false-positive ones in dMRI tractography ( @maierhein in @NatureComms). On average, it’s four times more likely these tracts are false than that they are true.
http://doi.org/10.1038/s41467-017-01285-x">https://doi.org/10.1038/s...
http://doi.org/10.1038/s41467-017-01285-x">https://doi.org/10.1038/s...
As another dear colleague told me, „the story was right (maybe given the MFB-rat research)“ and our field gladly welcomed the slMFB into the realms of a promising shiny new DBS target. At conferences, colleagues mentioned that potentially, effects could be attributed to the STN?
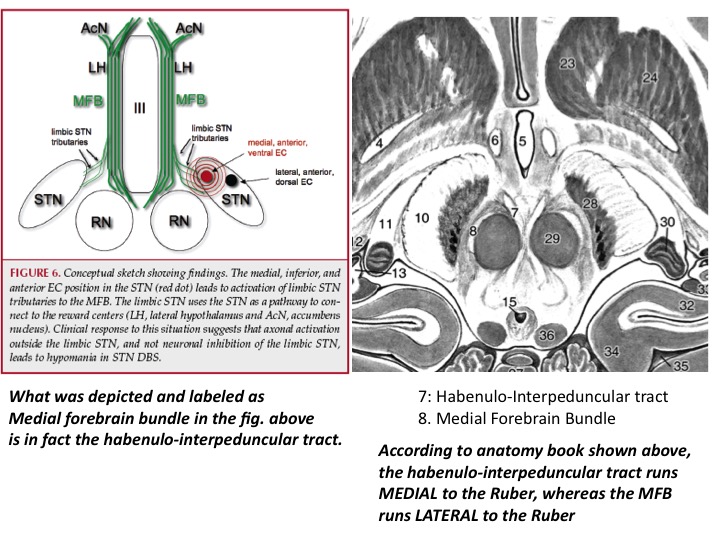
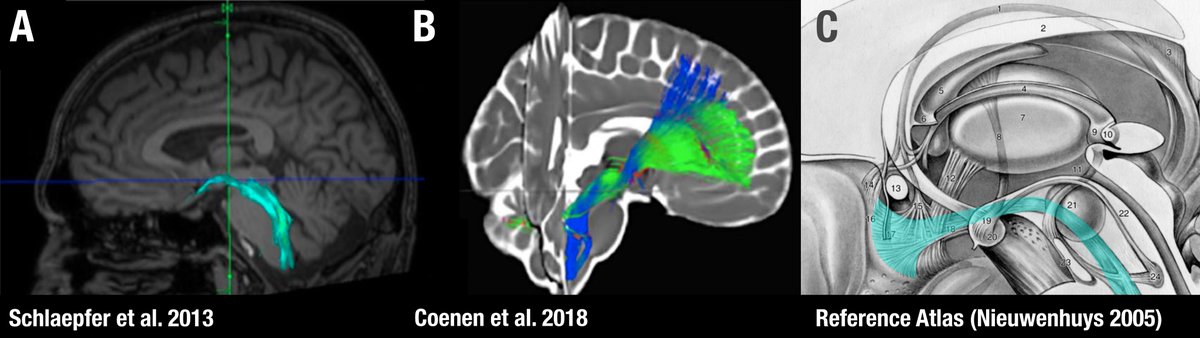
Already in the original publication, it remains a bit ambiguous whether the shown tract is really the MFB (which usually is lateral to the red nucleus e.g. as defined by Nieuwenhuys et al in their excellent anatomy handbook). This slide was shared w/ me by a colleague:
The main concept of stimulating the MFB to achieve positive reward could be doubtful. Usually, with high-frequency DBS, activity gets suppressed (as e.g. mentioned by Pierre Pollak in the same context in this podcast episode: http://stimulatingbrains.org/?p=224 ).">https://stimulatingbrains.org/...
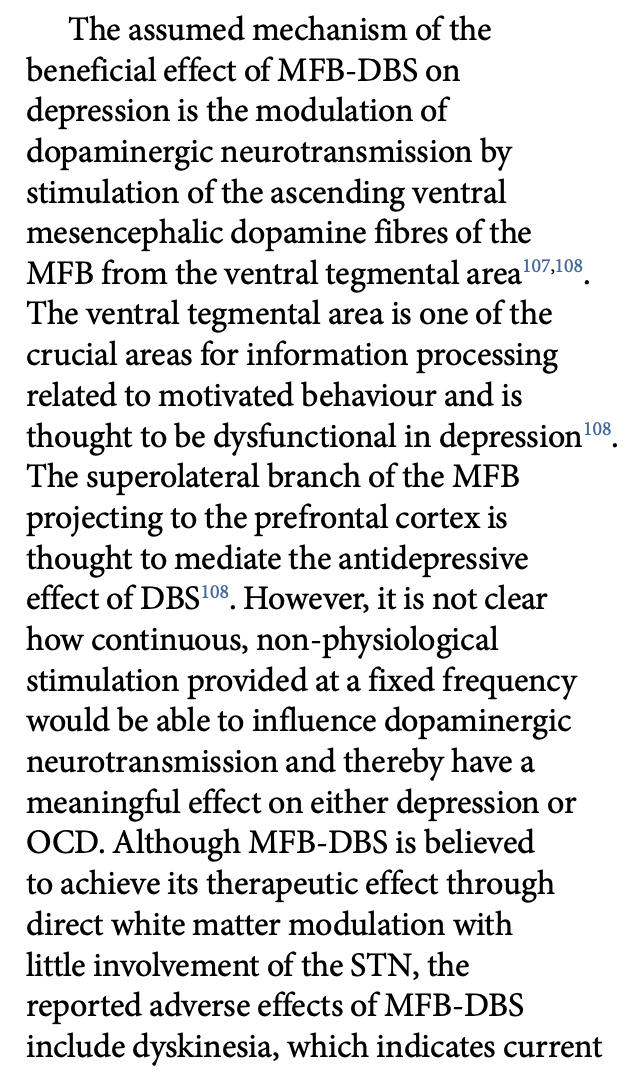
Bouthour et al. write: “However, it is not clear how continuous, non-physiological stimulation provided at a fixed frequency would be able to influence dopaminergic neurotransmission and thereby have a meaningful effect on either depression or OCD.”
http://doi.org/10.1038/s41582-019-0166-4">https://doi.org/10.1038/s...
http://doi.org/10.1038/s41582-019-0166-4">https://doi.org/10.1038/s...
Similarly, the (true) MFB primarily consists of dopaminergic and unmyelinated fibers. These are likely not activated using pulse-widths of 60 us and if anything should become depleted/blocked by 130 Hz.
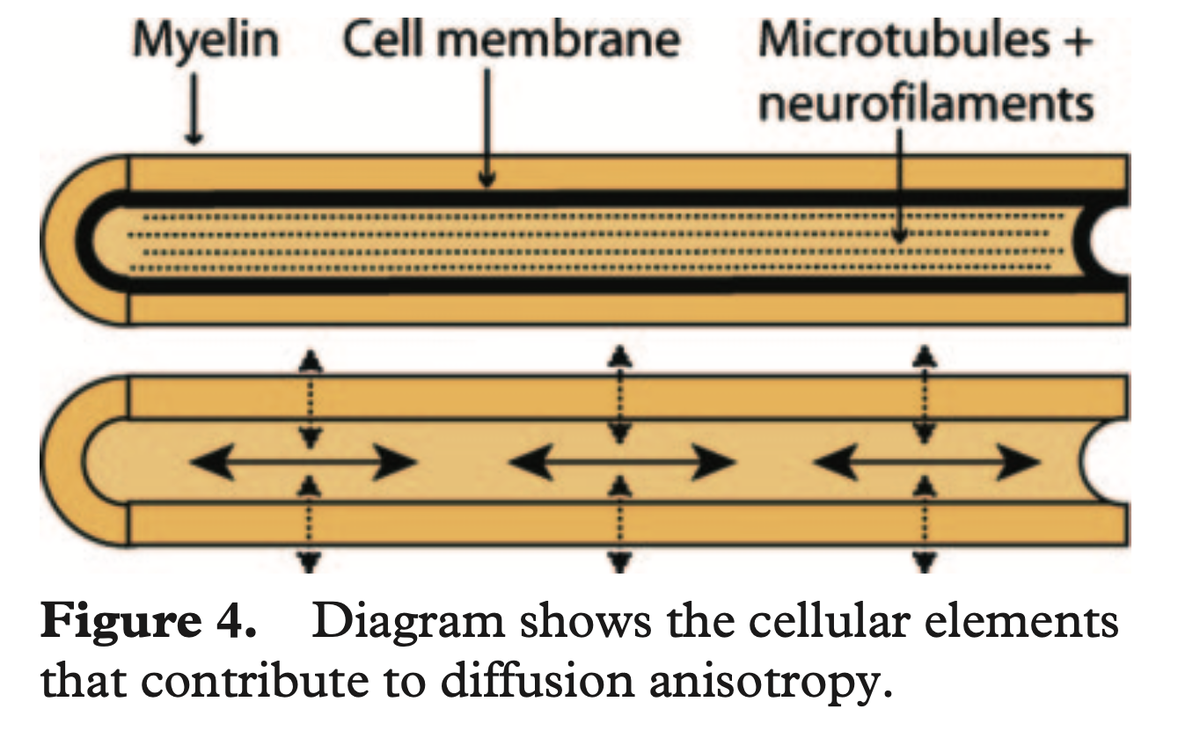
Moreover, such thin/unmyelinated fibers are very hard to see/differentiate with dMRI-tractography (more on that below).
(figure from Hagmann et al. 2006 http://doi.org/10.1148/rg.26si065510)">https://doi.org/10.1148/r...
(figure from Hagmann et al. 2006 http://doi.org/10.1148/rg.26si065510)">https://doi.org/10.1148/r...
So is it possible that instead of stimulating the medial forebrain bundle, studies that targeted the slMFB indeed modulated the nearby STN?
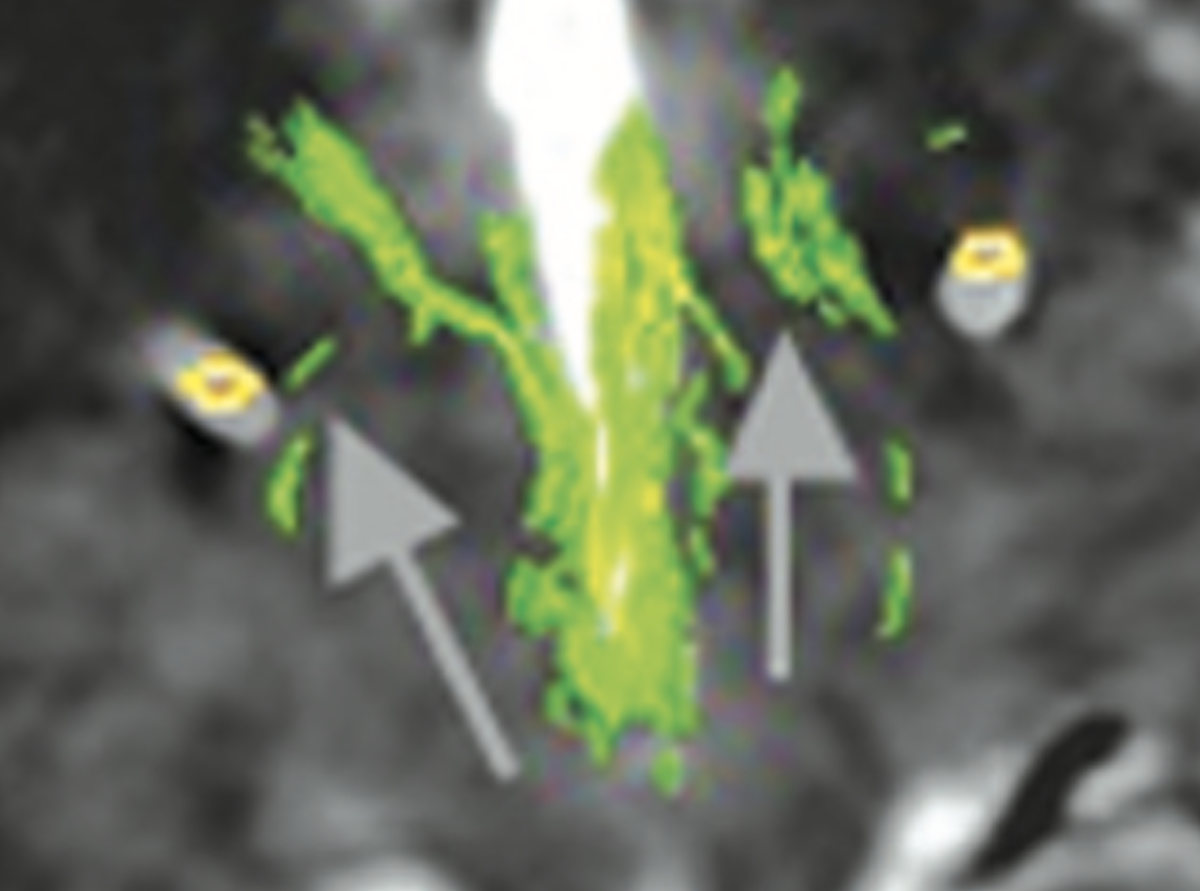
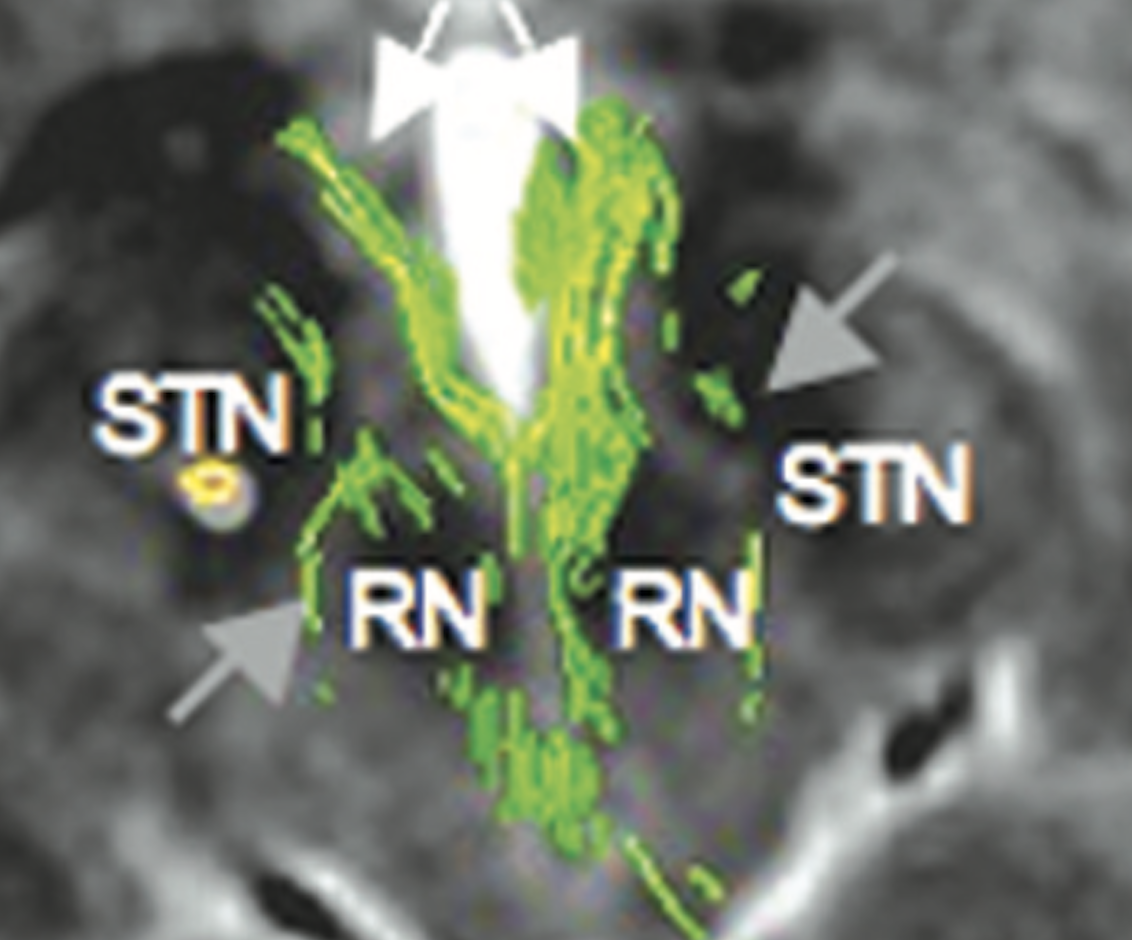
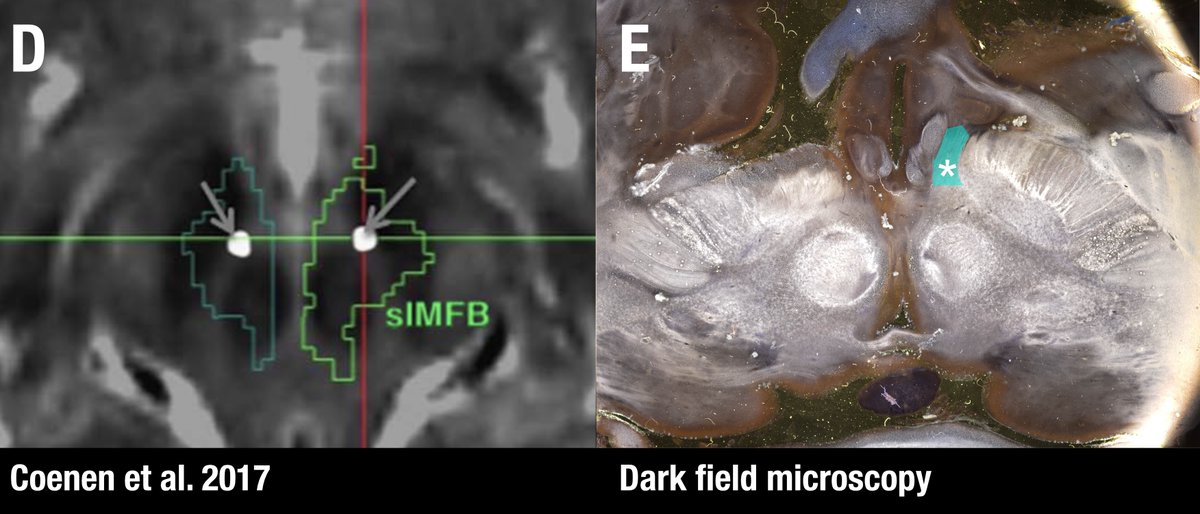
Sure. Below figure (Coenen et al. 2017) shows the slMFB covering most of the red nucleus and substantia nigra/STN. With the spread of VTAs we all know, it’s simply impossible to differentiate the anatomical substrate of DBS if structures are so close to each other.
So – should slMFB studies potentially be interpreted based as anteromedial STN studies?
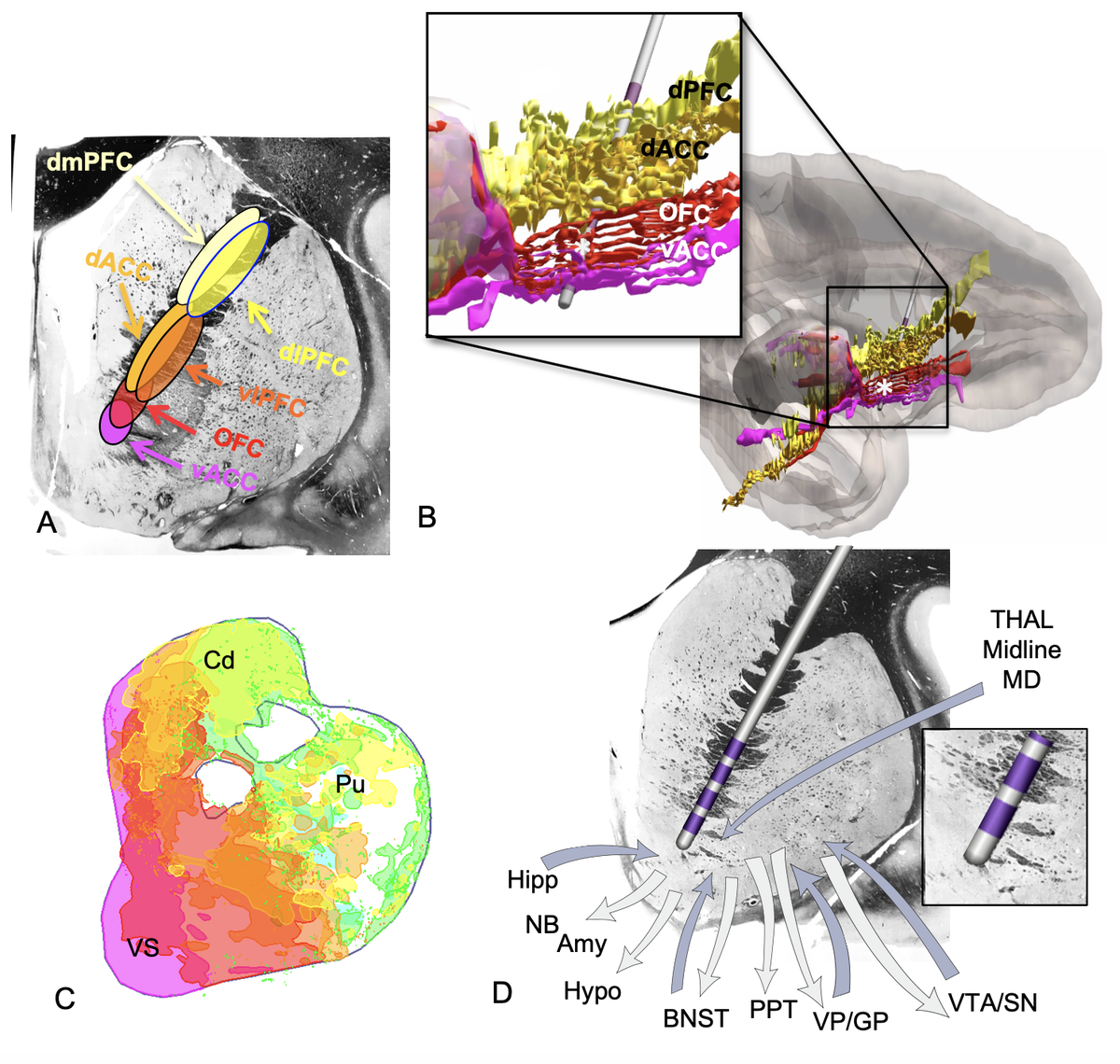
Yes! Remember, the STN receives input from the whole frontal cortex, including of course OFC and CG25.
Yes! Remember, the STN receives input from the whole frontal cortex, including of course OFC and CG25.
Also, remember this slide I just showed you. Is Apathy the Akinesia of the limbic system? Could it make sense that we may modulate it using DBS to the very avSTN?
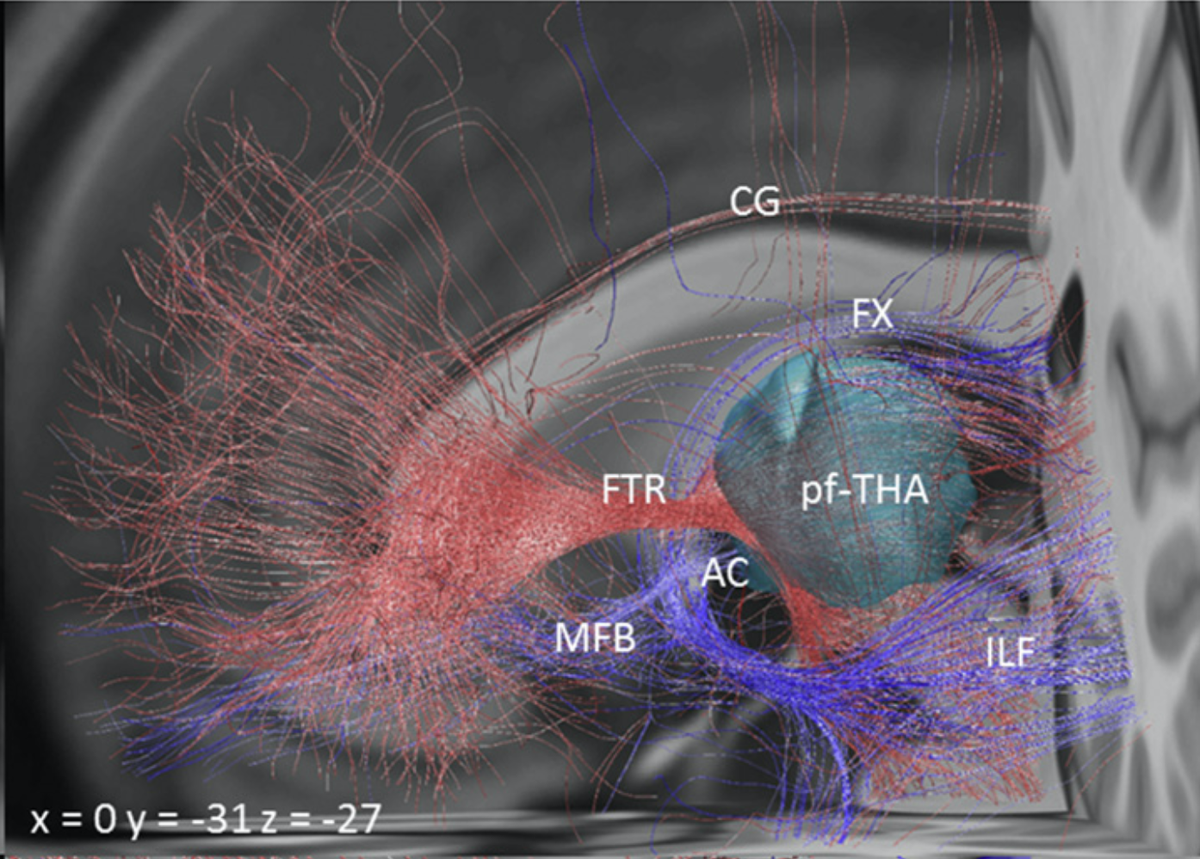
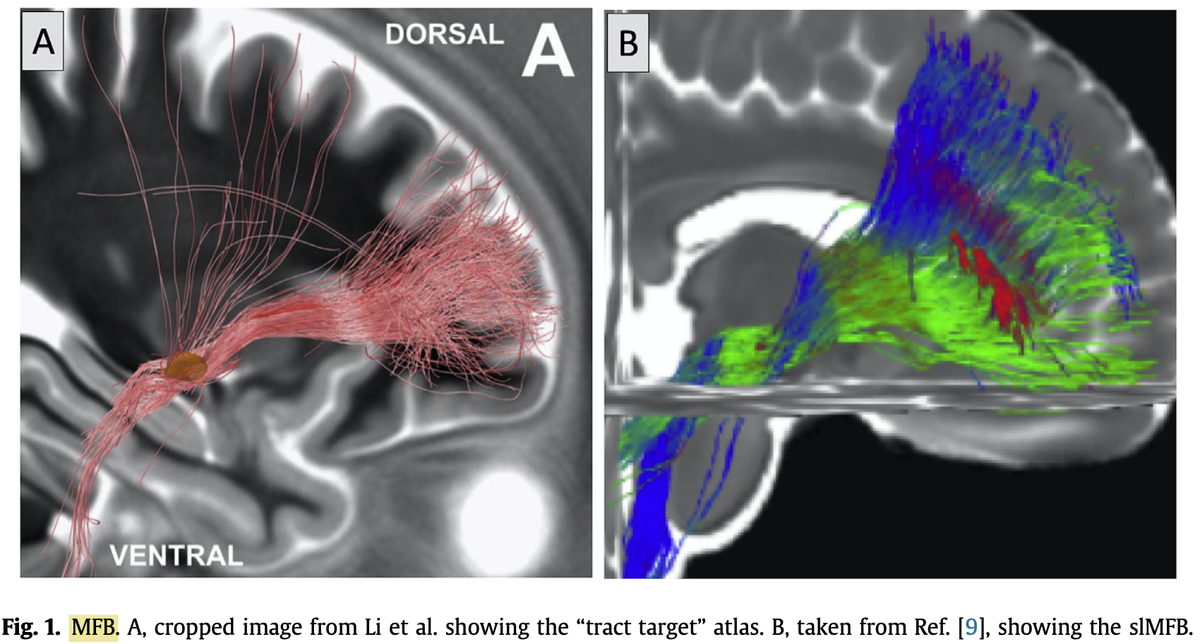
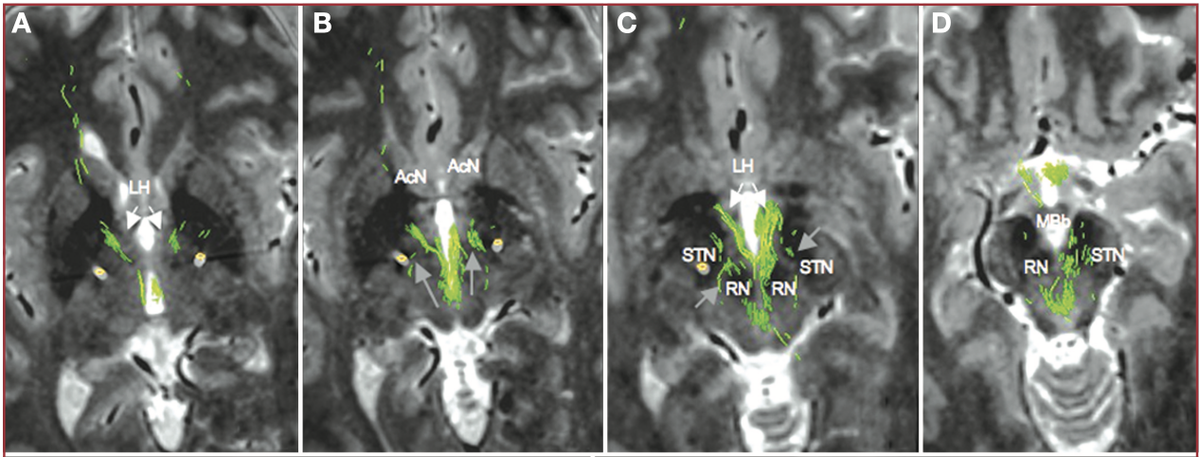
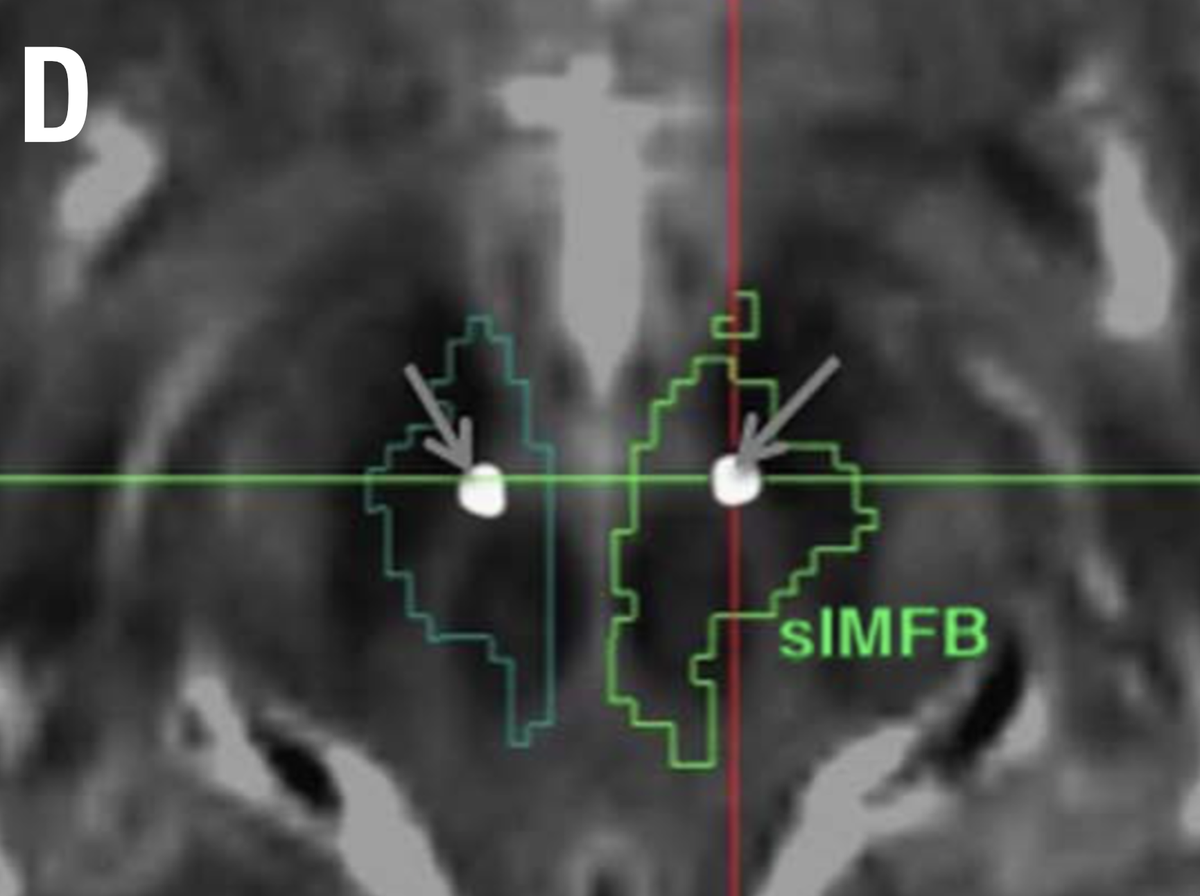
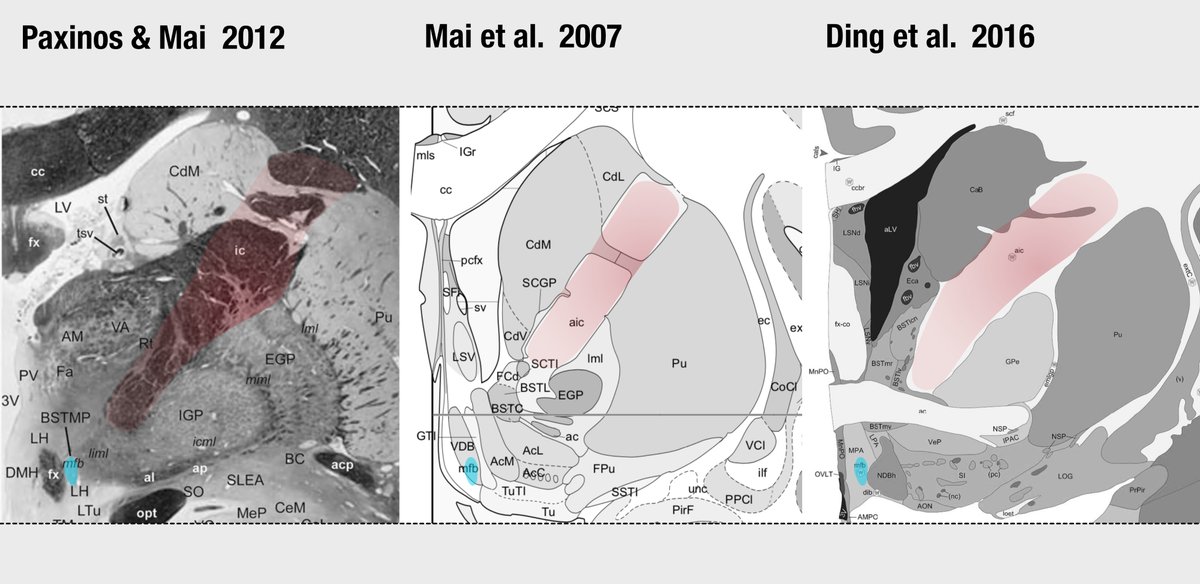
Another confusing issue is that the surgical slMFB target seems to have changed over time. Compare the two figures below. While the left one in principle corresponds to the anatomical definition of the MFB, the more recent definition does not. It runs within the internal capsule.
Contrasting the axial definition of the sl-MFB (Coenen et al. 2017) to a dark-field microscopy section kindly provided by @eduardo_alho shows tha the true (anatomical) MFB) traverses through the hypothalamus to the olfactory cortex.
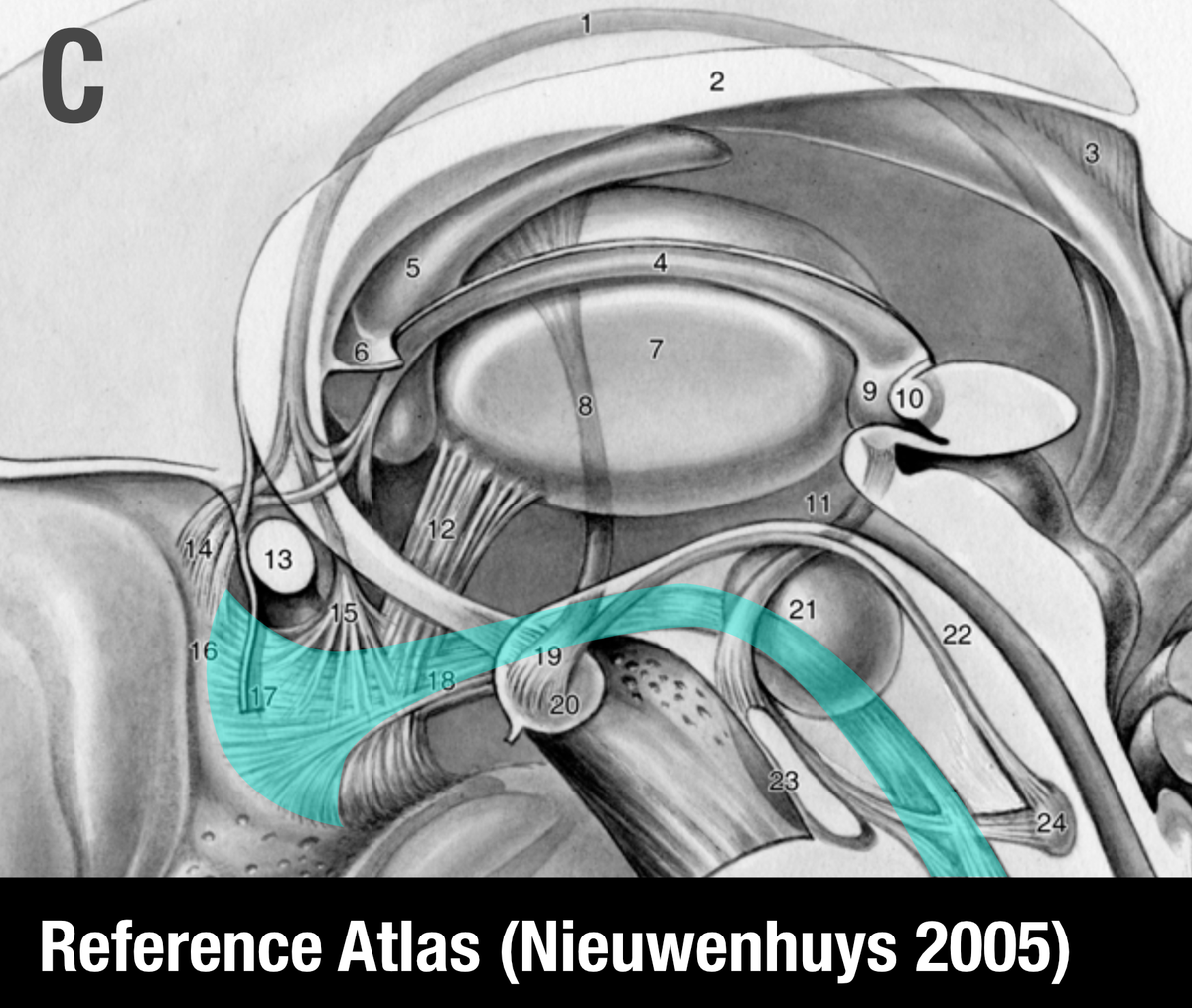
The MFB is a bundle that was predominantly characterized in the rat. One of the few people that published original work on it is Rudolf Nieuwenhuys – in his handbook, the MFB does not traverse within the anterior commissure.
Similar conclusions can be seen in other histological atlases. See coronal sections with the anterior limb of the internal capsule (red) and medial forebrain bundle (cyan) highlighted.
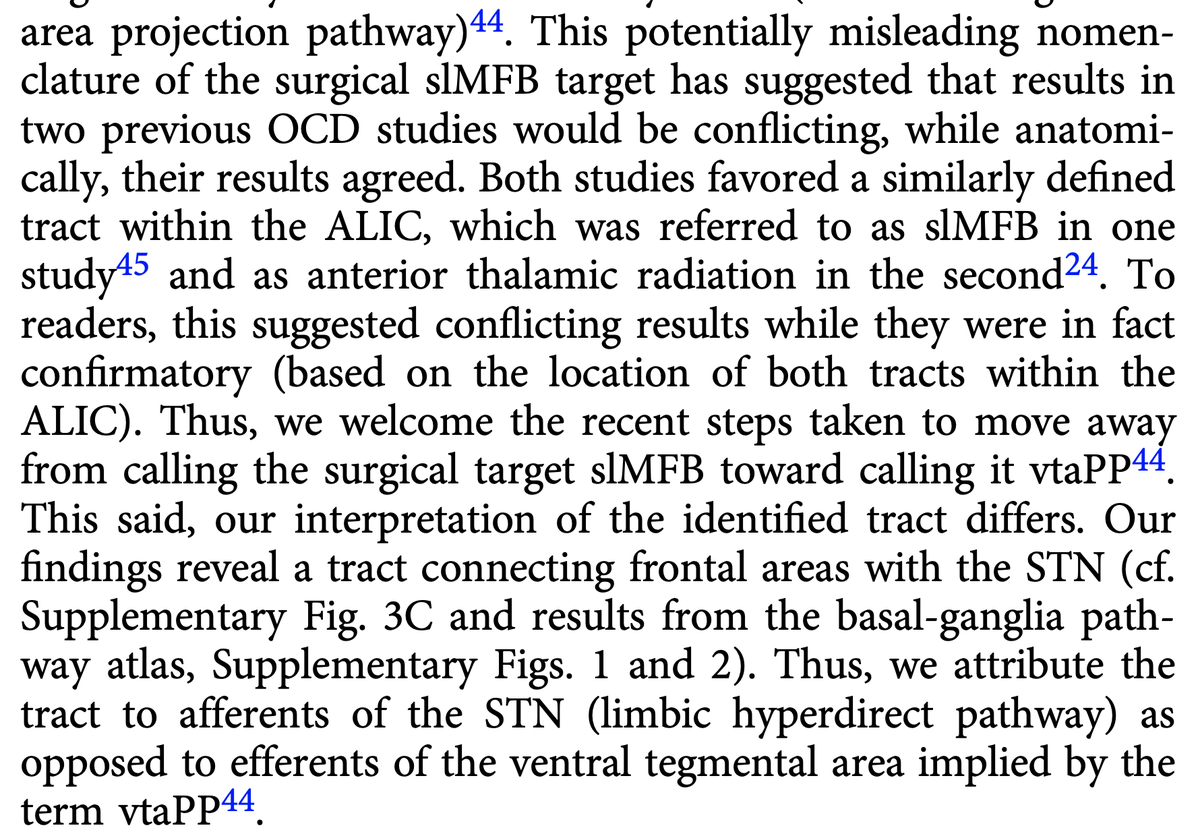
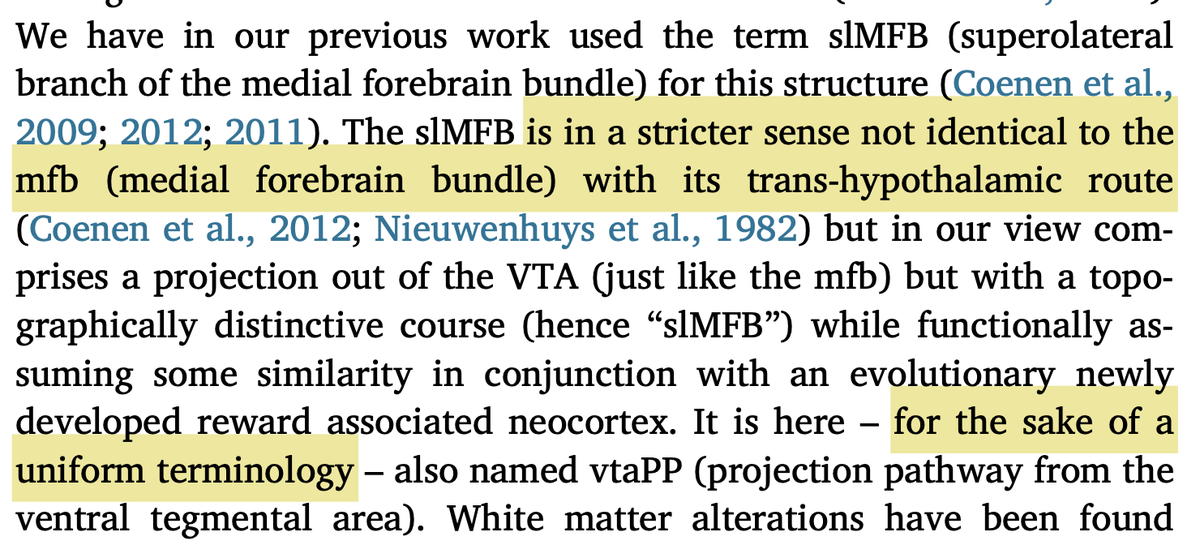
Fortunately, the original slMFB team has confirmed this issue and changed their nomenclature to vtaPP (more descriptive term as projection pathway from the VTA). This is great & I would like to congratulate the authors for this important step.  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> http://doi.org/10.1016/j.nicl.2020.102165">https://doi.org/10.1016/j...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> http://doi.org/10.1016/j.nicl.2020.102165">https://doi.org/10.1016/j...
However, as always this potentially misleading nomenclature got stuck and it& #39;s potentially hard to get it our of people& #39;s heads once it& #39;s in there. Read below for some examples:
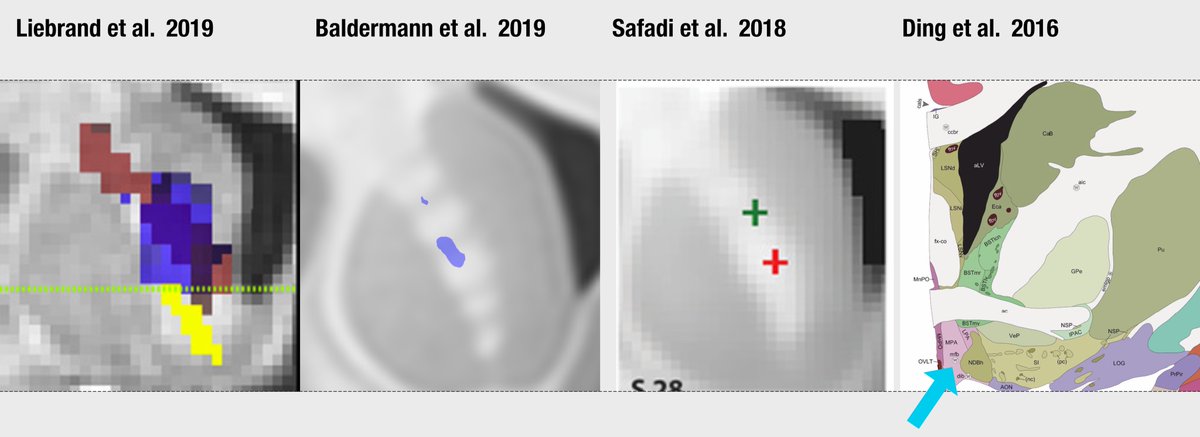
In two OCD-DBS studies, the (anatomical) conclusion of a "sweet tract" was the same.
Liebrand termed it slMFB (building on slMFB-DBS literature), Baldermann ALIC/anterior thalamic radiation (building on stereotactic surgical literature dating back to Leksell & Talairach in 1951)
Liebrand termed it slMFB (building on slMFB-DBS literature), Baldermann ALIC/anterior thalamic radiation (building on stereotactic surgical literature dating back to Leksell & Talairach in 1951)
In fact, the MFB is mentioned by Baldermann et al., as well – but as tract associated with negative clinical outcome.
(In hindsight, the tract shown here is probably also not the MFB but was defined as the MFB within a DTI-atlas).
(In hindsight, the tract shown here is probably also not the MFB but was defined as the MFB within a DTI-atlas).
If you read both studies, you think that they came to opposite conclusions (one  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> MFB, one
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> MFB, one  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👎" title="Thumbs down" aria-label="Emoji: Thumbs down"> MFB). However, their anatomical target (at A-P-level of the electrodes) is the same!
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👎" title="Thumbs down" aria-label="Emoji: Thumbs down"> MFB). However, their anatomical target (at A-P-level of the electrodes) is the same!
This is a perfect example that we need proper anatomical nomenclature.
This is a perfect example that we need proper anatomical nomenclature.
… – and good ways to make sure the tracts we define are correct. Alternative: Openly share your results as tract atlases in MNI. This is the most unambiguous and reproducible way I am aware of to avoid confusion.
Similarly, I fear that cross-species studies will conflate findings from the rat MFB (which is properly defined) and the human slMFB (which some people believe to be in the internal capsule). Haber et al write:
http://doi.org/10.1016/j.biopsych.2020.06.031">https://doi.org/10.1016/j...
http://doi.org/10.1016/j.biopsych.2020.06.031">https://doi.org/10.1016/j...
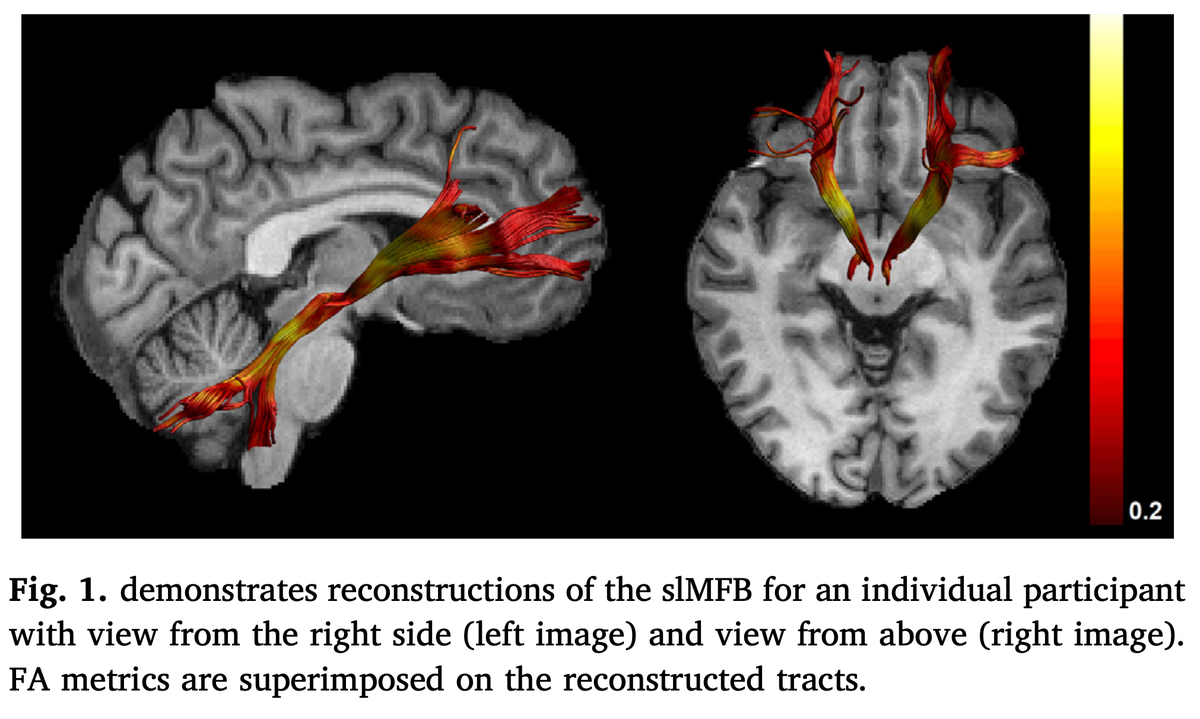
Finally, other authors have begun to mislabel the MFB, e.g. here in schizophrenia. The bundle shown is a beautiful internal capsule and even connects to the cerebellum. http://doi.org/10.1016/j.nicl.2019.102044">https://doi.org/10.1016/j...
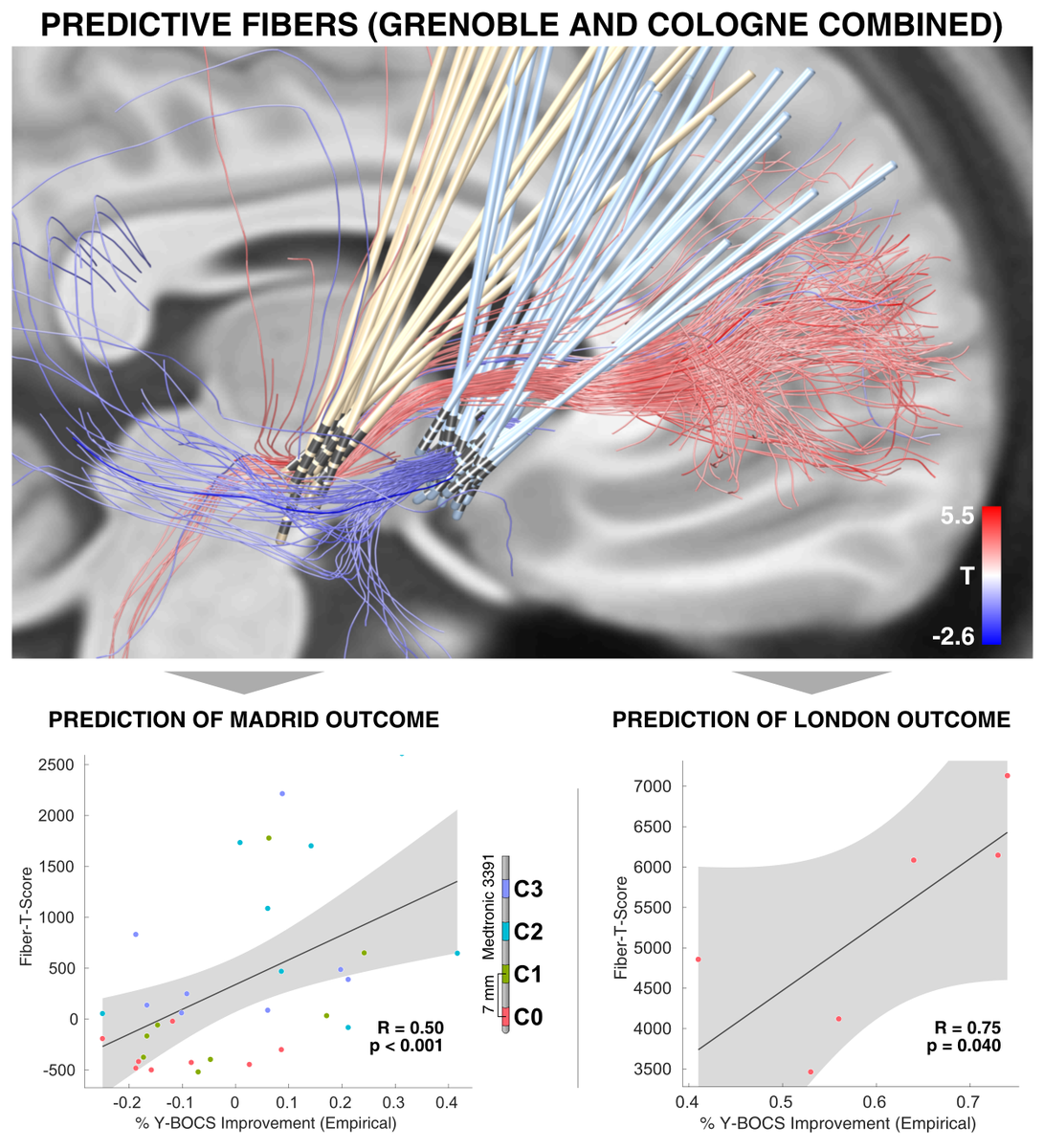
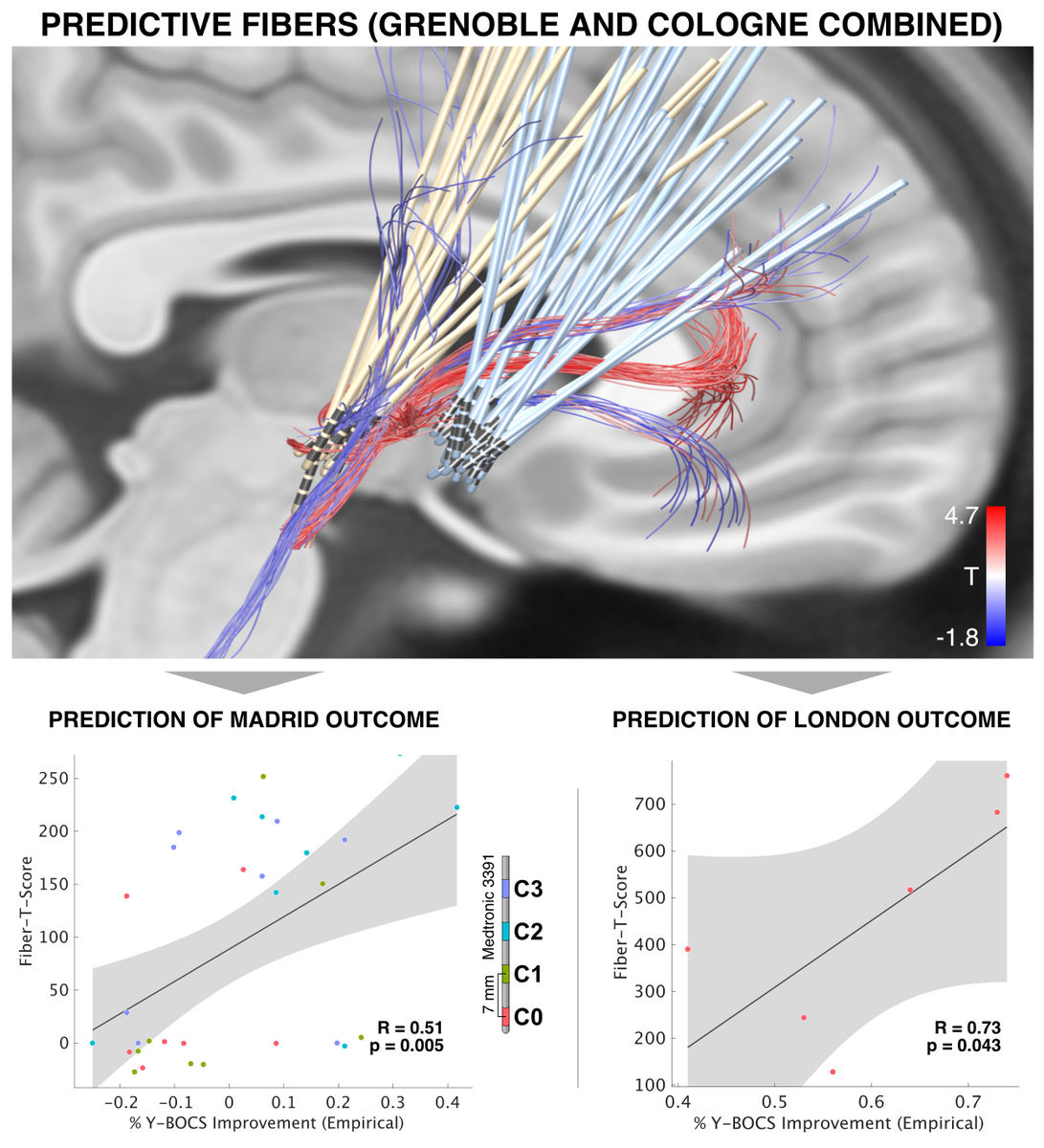
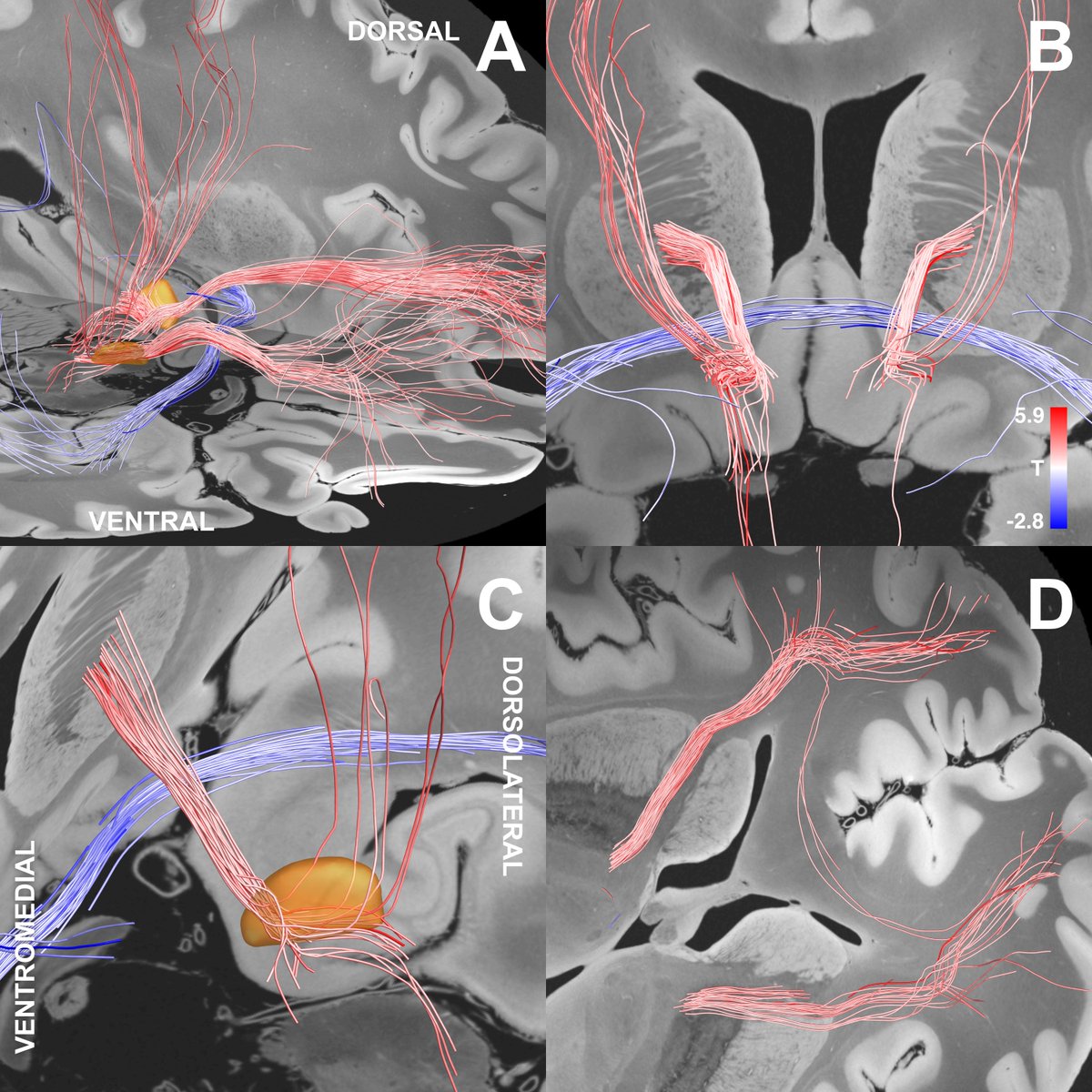
These and similar stories made me very weary about making mistakes with anatomical nomenclature. In our own OCD-DBS study ( @NingfeiL et al. @NatureComms), we asked four anatomists to confirm the nomenclature..
https://www.researchgate.net/publication/342658760_A_unified_connectomic_target_for_deep_brain_stimulation_in_obsessive-compulsive_disorder">https://www.researchgate.net/publicati...
https://www.researchgate.net/publication/342658760_A_unified_connectomic_target_for_deep_brain_stimulation_in_obsessive-compulsive_disorder">https://www.researchgate.net/publicati...
Our study identified a specific sub-bundle of the anterior limb of the internal capsule to be associated with optimal clinical outcome.
It was able to predict clinical improvement following DBS for OCD in 50 patients from four centers
https://www.researchgate.net/publication/342658760_A_unified_connectomic_target_for_deep_brain_stimulation_in_obsessive-compulsive_disorder">https://www.researchgate.net/publicati...
It was able to predict clinical improvement following DBS for OCD in 50 patients from four centers
https://www.researchgate.net/publication/342658760_A_unified_connectomic_target_for_deep_brain_stimulation_in_obsessive-compulsive_disorder">https://www.researchgate.net/publicati...
A preprint of our study had been criticised by Coenen and colleagues: The bundle we show should be called MFB.
http://doi.org/10.1016/j.brs.2019.07.014">https://doi.org/10.1016/j...
http://doi.org/10.1016/j.brs.2019.07.014">https://doi.org/10.1016/j...
Obviously, we do show the same bundle – which is reason for celebration!
However, our anatomical label is different. Moreover, our interpretation of findings is different.
Namely, we attribute effects to the limbic hyperdirect pathway of the STN.
However, our anatomical label is different. Moreover, our interpretation of findings is different.
Namely, we attribute effects to the limbic hyperdirect pathway of the STN.
This claim is a hypothesis, as well, backed by but a bit of empirical data.
First, we reproduced connectome findings using a holographic atlas of the brain which shows an accurate description of the limbic hyperdirect pathway.
First, we reproduced connectome findings using a holographic atlas of the brain which shows an accurate description of the limbic hyperdirect pathway.
Second, the tract we isolated does indeed traverse exactly within the anteromedial STN. Again – this is using dMRI-tractography for anatomical conclusions and should not be overinterpreted.
Finally, DBS to the amSTN works well for OCD (there are many studies). Secondly, Lesions to the ALIC and dACC work well for OCD. DBS to the ALIC works well.
This exact set of connections is part of the classical CSTC model for OCD.
This exact set of connections is part of the classical CSTC model for OCD.
If you are interested about this topic, I can really recommend the excellent article by Suzanne & team which is a perfect and timely overview about anatomical connections of DBS for OCD.
http://doi.org/10.1016/j.biopsych.2020.06.031">https://doi.org/10.1016/j...
http://doi.org/10.1016/j.biopsych.2020.06.031">https://doi.org/10.1016/j...

 Read on Twitter
Read on Twitter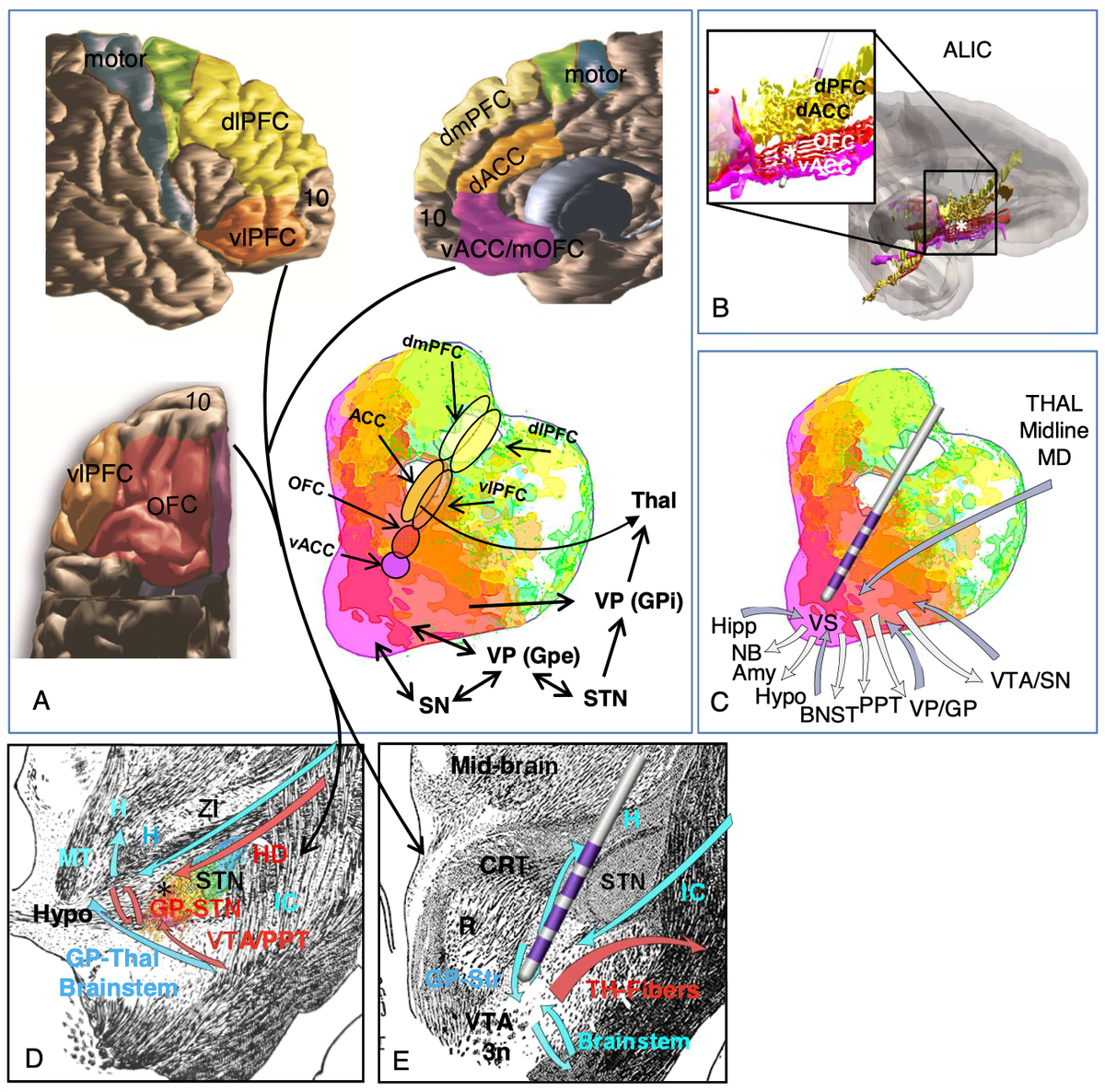
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://doi.org/10.1016/j..." title="Fortunately, the original slMFB team has confirmed this issue and changed their nomenclature to vtaPP (more descriptive term as projection pathway from the VTA). This is great & I would like to congratulate the authors for this important step. https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://doi.org/10.1016/j..." class="img-responsive" style="max-width:100%;"/>
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://doi.org/10.1016/j..." title="Fortunately, the original slMFB team has confirmed this issue and changed their nomenclature to vtaPP (more descriptive term as projection pathway from the VTA). This is great & I would like to congratulate the authors for this important step. https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙌" title="Raising hands" aria-label="Emoji: Raising hands">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://doi.org/10.1016/j..." class="img-responsive" style="max-width:100%;"/>