1) 1077-person trial by Oxford ChAdOx1 show immune response in 14 days. Booster improves at 28 days.
2) 602-person trial in Wuhan: immune response within 14 days. No serious side effects.
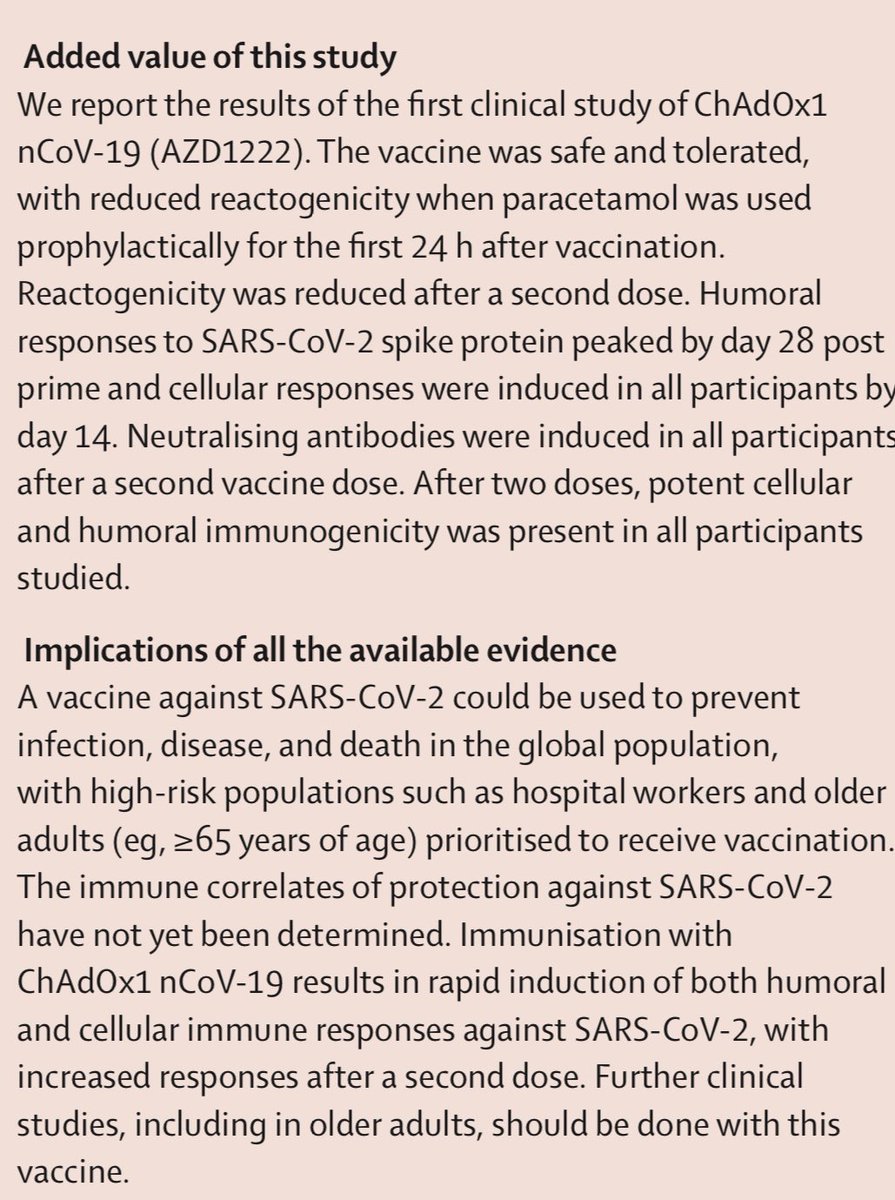
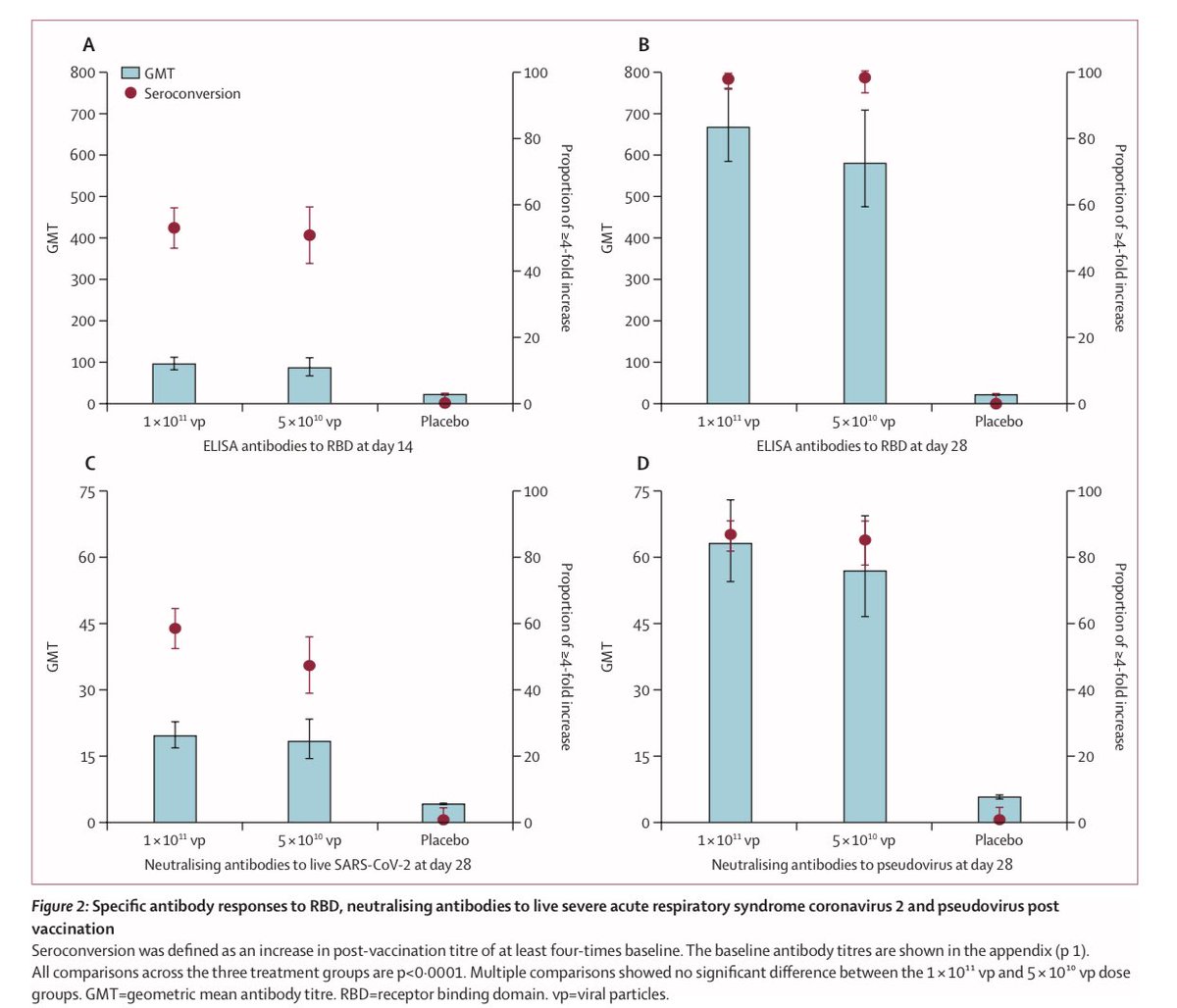
2) Oxford trial report. No serious adverse effects. Acetaminophen, aka paracetamol, reduced any mild effects. Neutralizing antibodies induced in all patients after second dose. Neutralising antibody responses correlated strongly with antibody levels
https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620316044.pdf">https://marlin-prod.literatumonline.com/pb-assets...
https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620316044.pdf">https://marlin-prod.literatumonline.com/pb-assets...
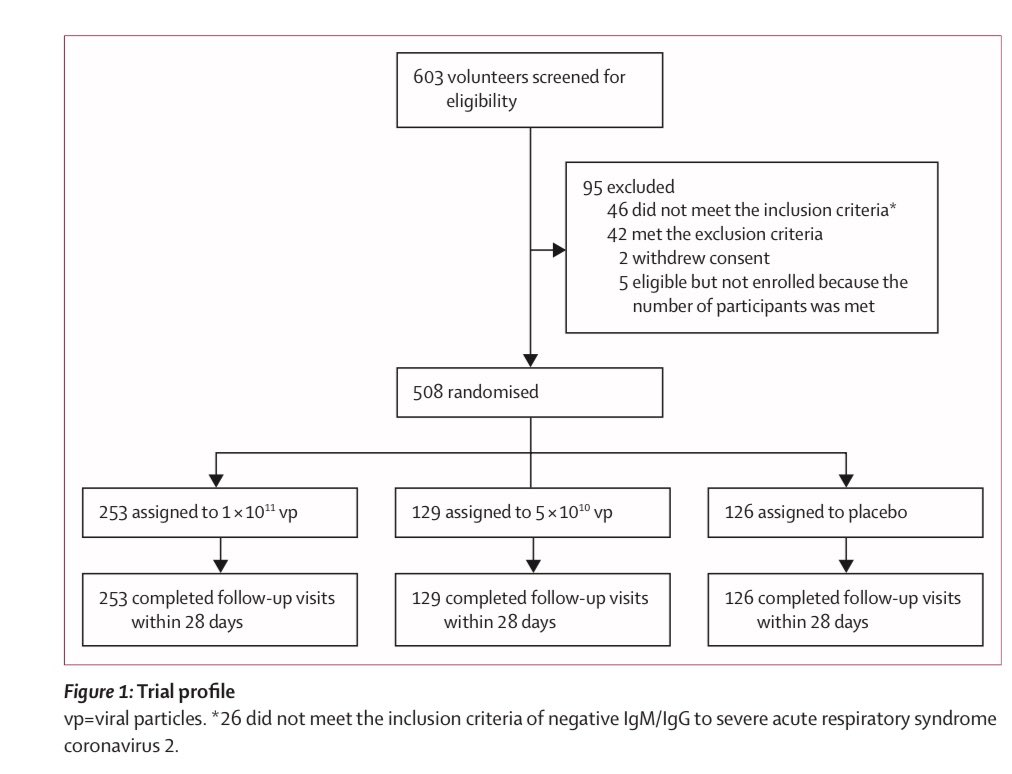
3) Wuhan adenovirus trial with 508 randomized participants of a single dose, half dose, or placebo. Seroconversion (antibody) development similar with full or half dose. Single dose was sufficient. https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620316056.pdf">https://marlin-prod.literatumonline.com/pb-assets...
4) In the Wuhan trial, At both 14 and 28 days, both antibodies and neutralizing antibodies much higher than placebo. The half dose (middle dose, however you call it) was almost just as effective as the higher dose. T-cell response similar story.
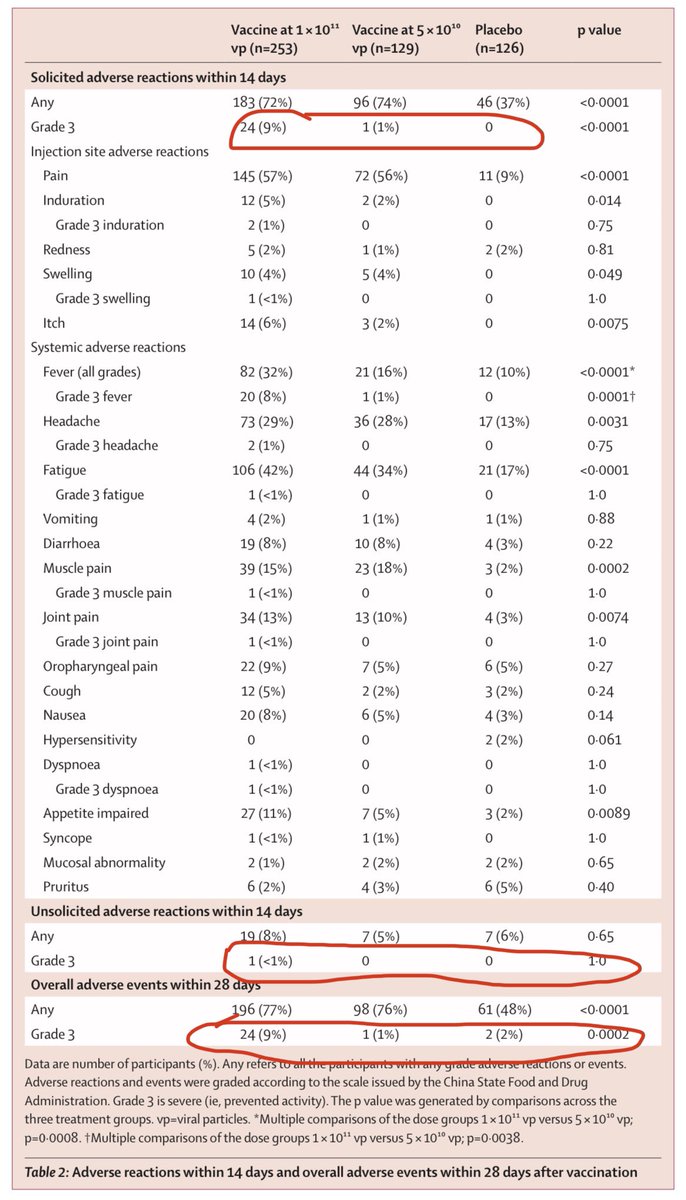
5) Adverse reactions in Wuhan trial is pretty low- and especially the medium half dose seems to have almost little to no “grade 3” reaction (that prevents activity) above placebo. Recall half dose almost as effective as the higher dose. No serious adverse reported in any group.
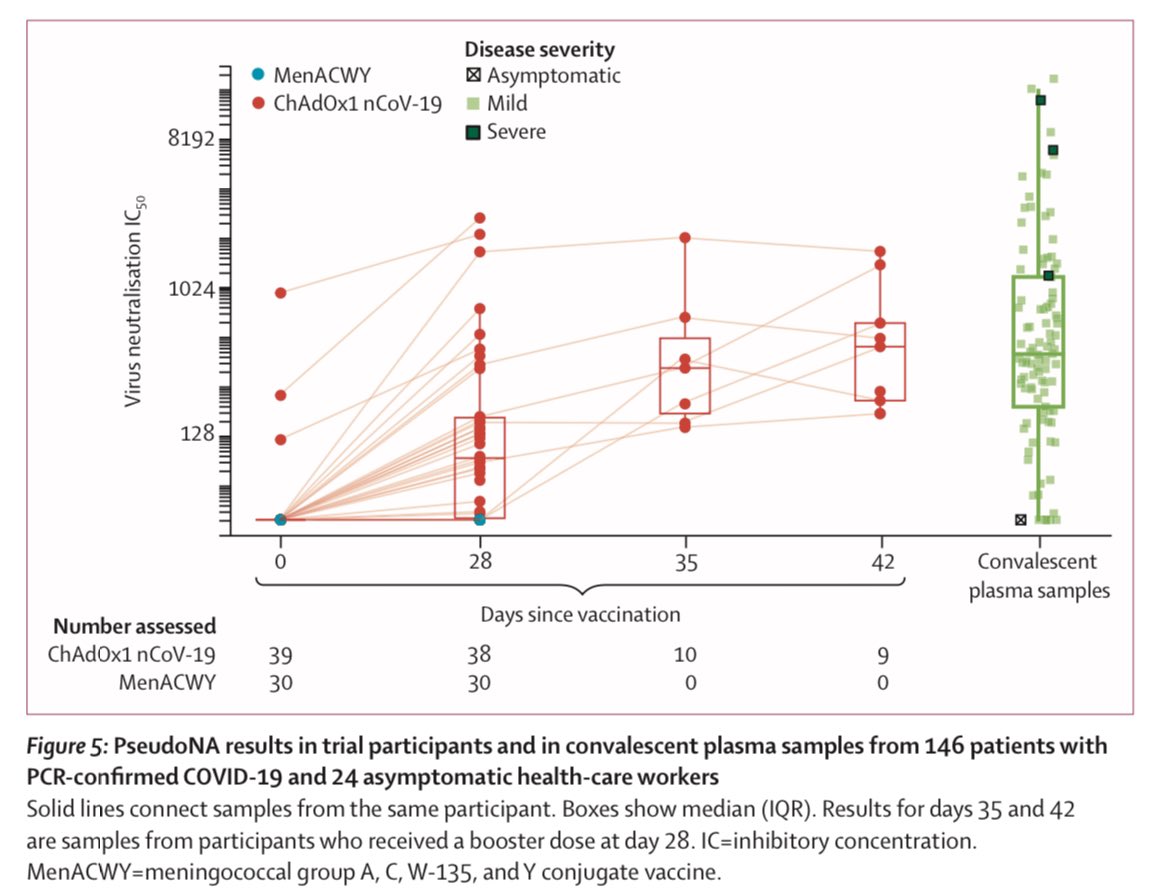
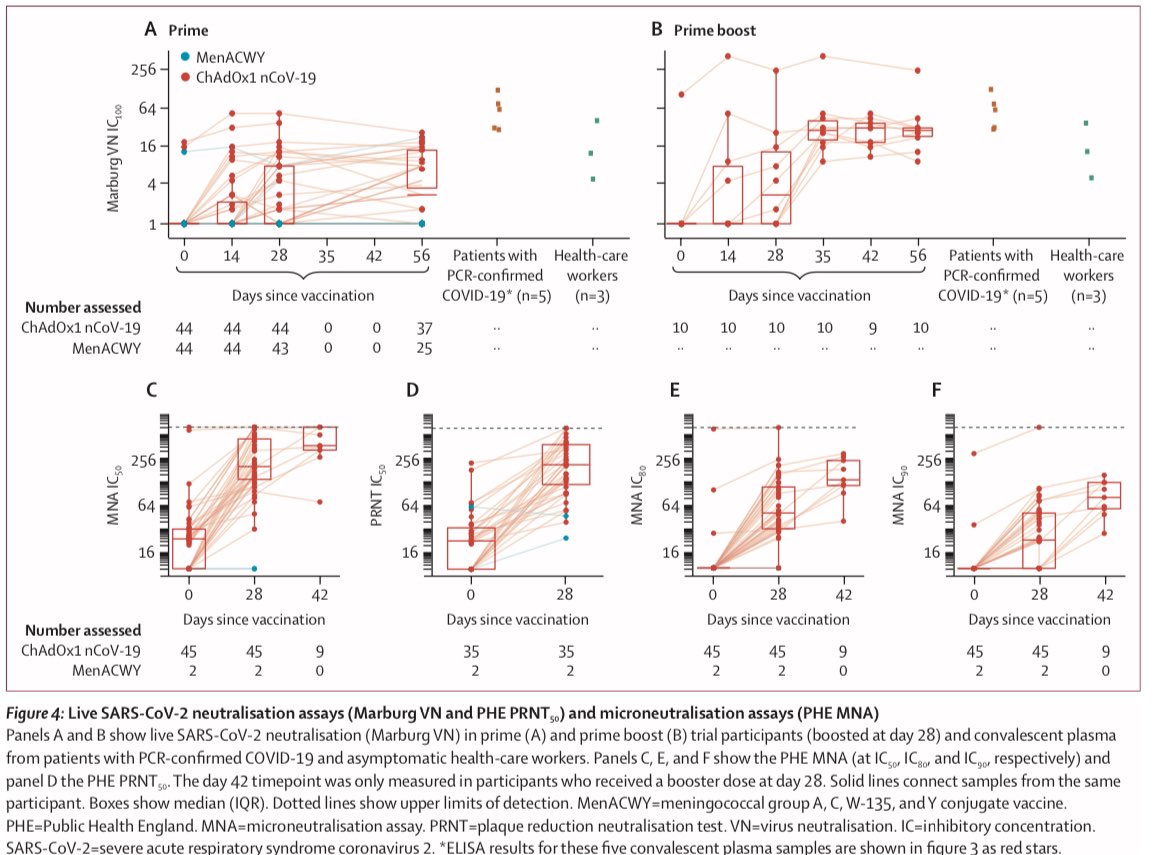
6) Back to the Oxford ChAdOx1 vaccine for #COVID19, the vaccine and its booster both outperformed versus the control (blue) for both IgG antibodies and in a variety of neutralizing assays.
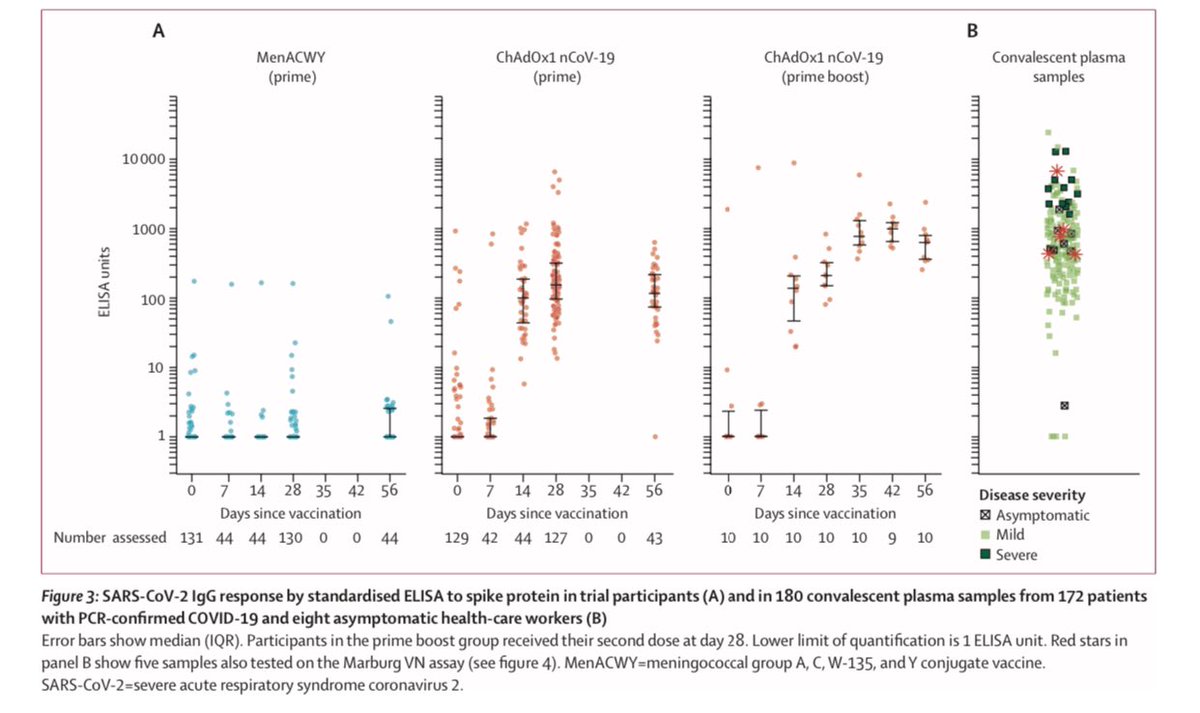
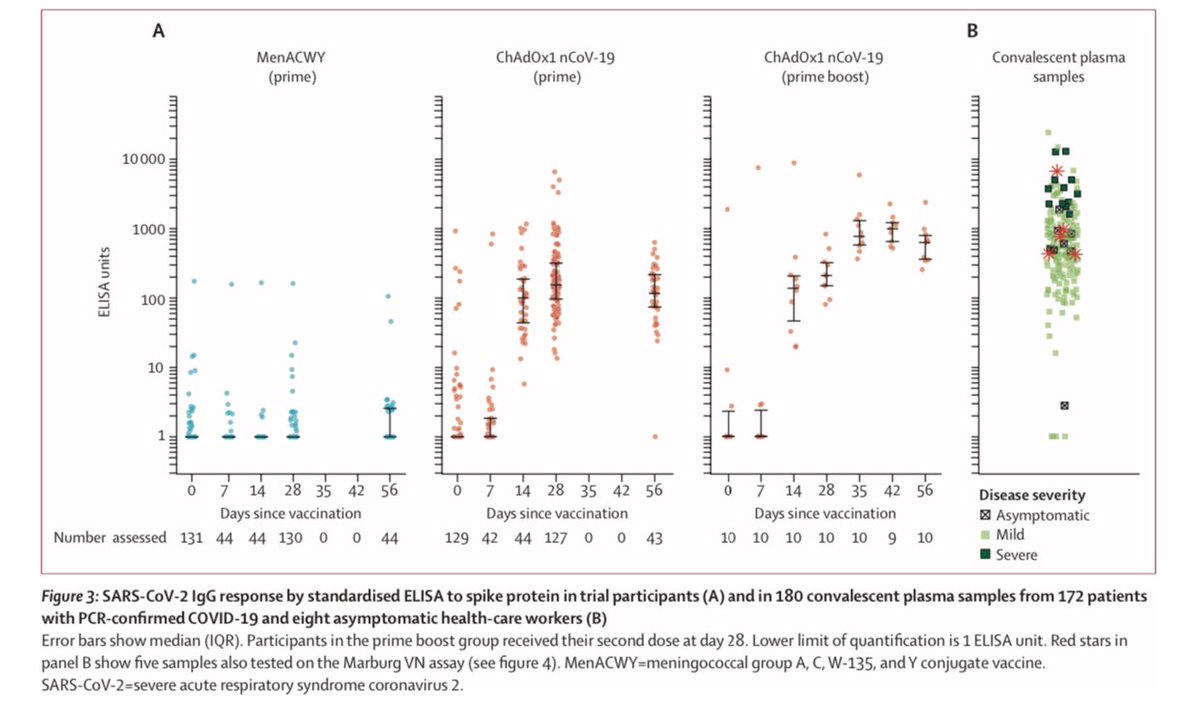
7) In Oxford trial, antibodies peaked at 28 days among those who got the single dose. It was very decent response, though the booster at 28 days did further increase it to the levels seen in other COVID patients’ convalescent plasma. Regardless, single dose still good to day 56.
8) I’m waiting to get thru the trial reports. But safety looks good in both trials. Here is an accompanying commentary on both trials for a semi-lay explanation: https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620316111.pdf">https://marlin-prod.literatumonline.com/pb-assets...
9) Mini-Summary: Both 2 early phase trials are very encouraging in preliminary efficacy & safety so far. But they are not ready for general population yet—we still need Phase 3 larger trials, to study efficacy/safety in more subgroups. Overall, they augur well for what’s to come!
10) StatNews has a good summary and background on the Oxford vaccine results and its manufacturing partnership with AstraZeneca. https://www.statnews.com/2020/07/20/study-provides-first-glimpse-of-efficacy-of-oxford-astrazeneca-covid-19-vaccine/">https://www.statnews.com/2020/07/2...
11) To be clear—results bode well for long term immunity. “Andrew Pollard of Oxford, lead author, said vaccine is intended to induce both types of responses. “We hope this means immune system will remember the virus, so that our vaccine will protect people for an extended period”
12) The vaccine has already entered large-scale tests, known as Phase 3 trials, aimed at proving its efficacy. It will also be part of the large trials being conducted in the U.S. as part of the government-run program known as Operation Warp Speed.
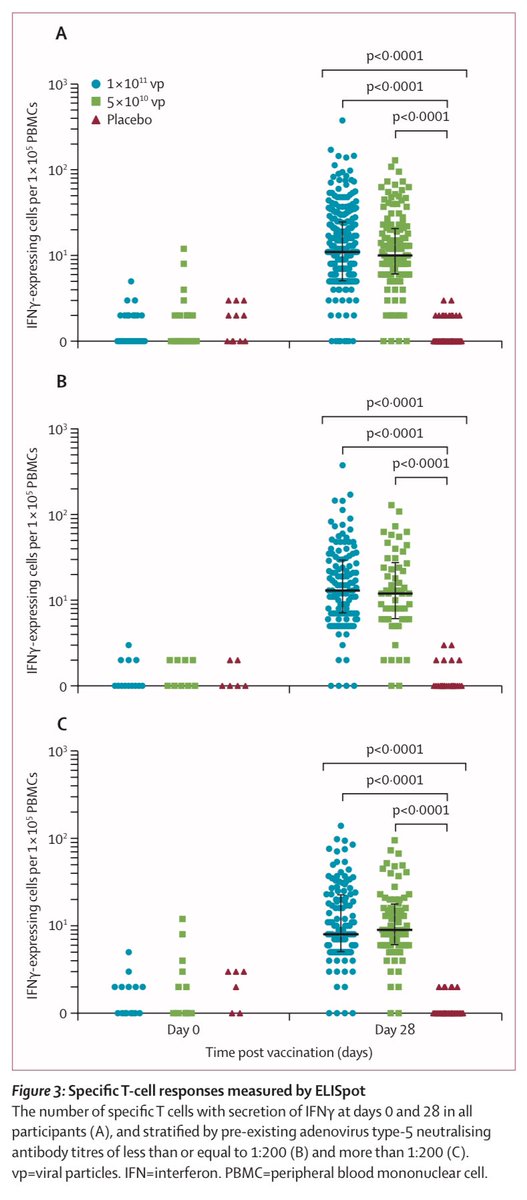
13) And result do show “both types” of immunity - humoral (antibody) and cellular (T cell) response. Neutralising antibodies were generated in more than 90% of participants across different assays. Responses were sustained up to 56 days of observation.
14) ...and a small non-randomly selected, second-dose boosted subset showed strong neutralising responses.
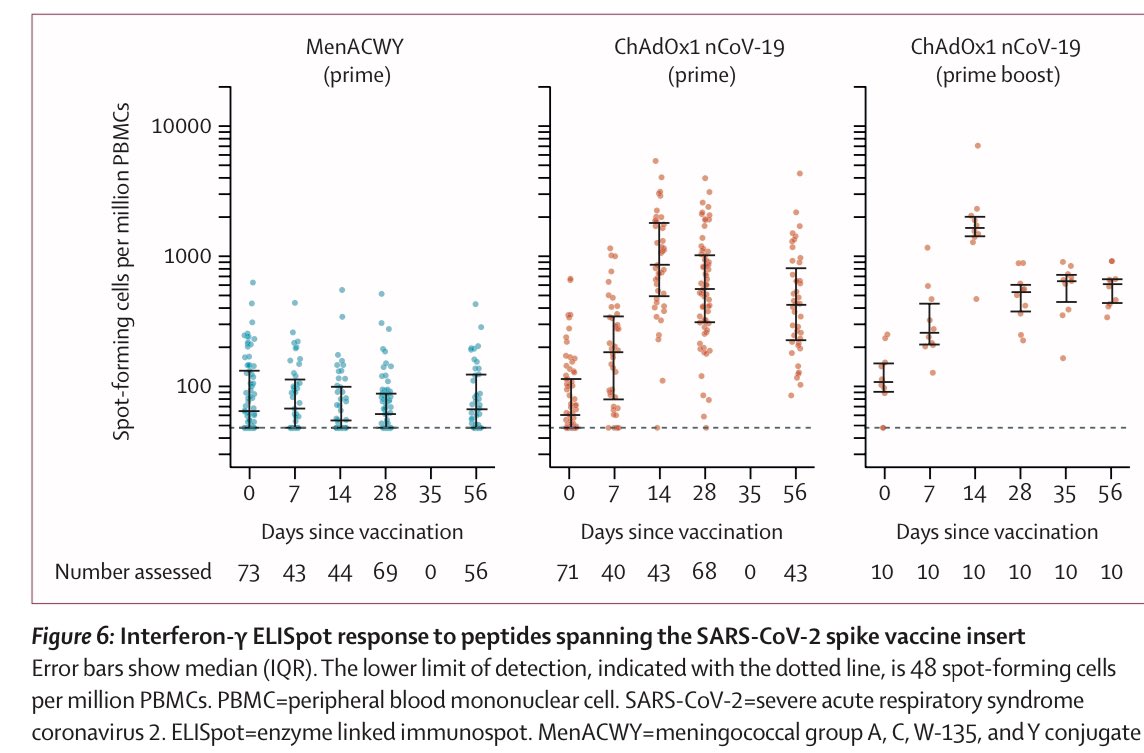
And Importantly, T-cell responses were induced in all participants.
This is why Andrew Pollard is hopeful for long term immunity cuz it improves both these immunity types.
And Importantly, T-cell responses were induced in all participants.
This is why Andrew Pollard is hopeful for long term immunity cuz it improves both these immunity types.
15) WHO statement: "We do welcome the study and congratulate our colleagues at the Oxford University& #39;s Jenner Institute”, says @DrMikeRyan. "This is a positive result but again there is a long way to go."
16) I explain more about T cell immunity in this earlier thread last week about the Oxford vaccine before results came out today. https://twitter.com/drericding/status/1283799377845395456?s=21">https://twitter.com/drericdin... https://twitter.com/drericding/status/1283799377845395456">https://twitter.com/drericdin...
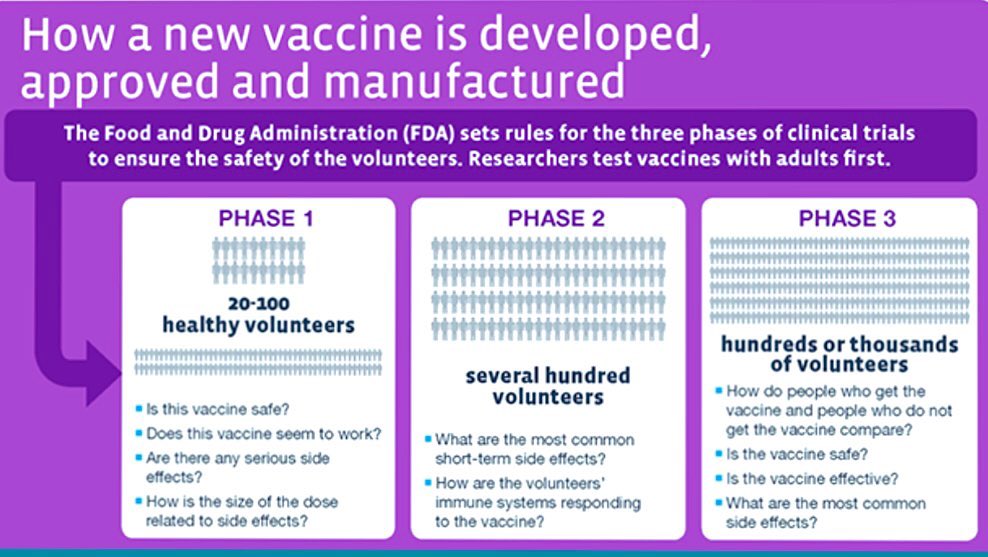
17) What does all the major clinical trial phases mean? Here is an info graphic. The Oxford trial was a phase 2 , while the Wuhan was a combo Phase 1/2. We still need phase 3 trials, which are underway. Oxford vaccine’s phase 3 ongoing in Brazil.
Special thanks to @richardhorton1’s team for carefully reviewing these 2 vaccine trials.
18) A reminder why vaccines are important. https://twitter.com/drericding/status/1285281389655490568?s=21">https://twitter.com/drericdin... https://twitter.com/drericding/status/1285281389655490568">https://twitter.com/drericdin...
19) Another good explainer on the Oxford vaccine via BBC. https://www.bbc.com/news/uk-53469839">https://www.bbc.com/news/uk-5...
20) I want to reemphasize that measuring immune responses in these two trials is not the same as proving efficacy for actually preventing infection from #covid19 yet. That is what Phase 3 is for. And it’s underway. The more rampant epidemic is, faster we get results. Sad reality.
21) Meantime, these results give us hope. And we are hopeful given that Oxford’s ChAdOx1 vaccine system has been previous shown to be successful for providing immunity to MERS coronavirus in macaques. https://advances.sciencemag.org/content/6/24/eaba8399">https://advances.sciencemag.org/content/6...

 Read on Twitter
Read on Twitter