1/16
Why does uremia cause platelet dysfunction (i.e., what are "uremic platelets")?
The association between kidney disease and bleeding has been observed for centuries and remains incompletely understood.
Let& #39;s have a look at platelets, bleeding, and a few of those humours.
Why does uremia cause platelet dysfunction (i.e., what are "uremic platelets")?
The association between kidney disease and bleeding has been observed for centuries and remains incompletely understood.
Let& #39;s have a look at platelets, bleeding, and a few of those humours.
2/
Just about every aspect of primary hemostasis is affected in uremia. For this thread, I& #39;ll focus on how toxins disrupt:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Adhesion (attachment of platelets to exposed subendothelial collagen)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Adhesion (attachment of platelets to exposed subendothelial collagen)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Aggregation (attachment of platelets to each other) https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Aggregation (attachment of platelets to each other) https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
Just about every aspect of primary hemostasis is affected in uremia. For this thread, I& #39;ll focus on how toxins disrupt:
3/
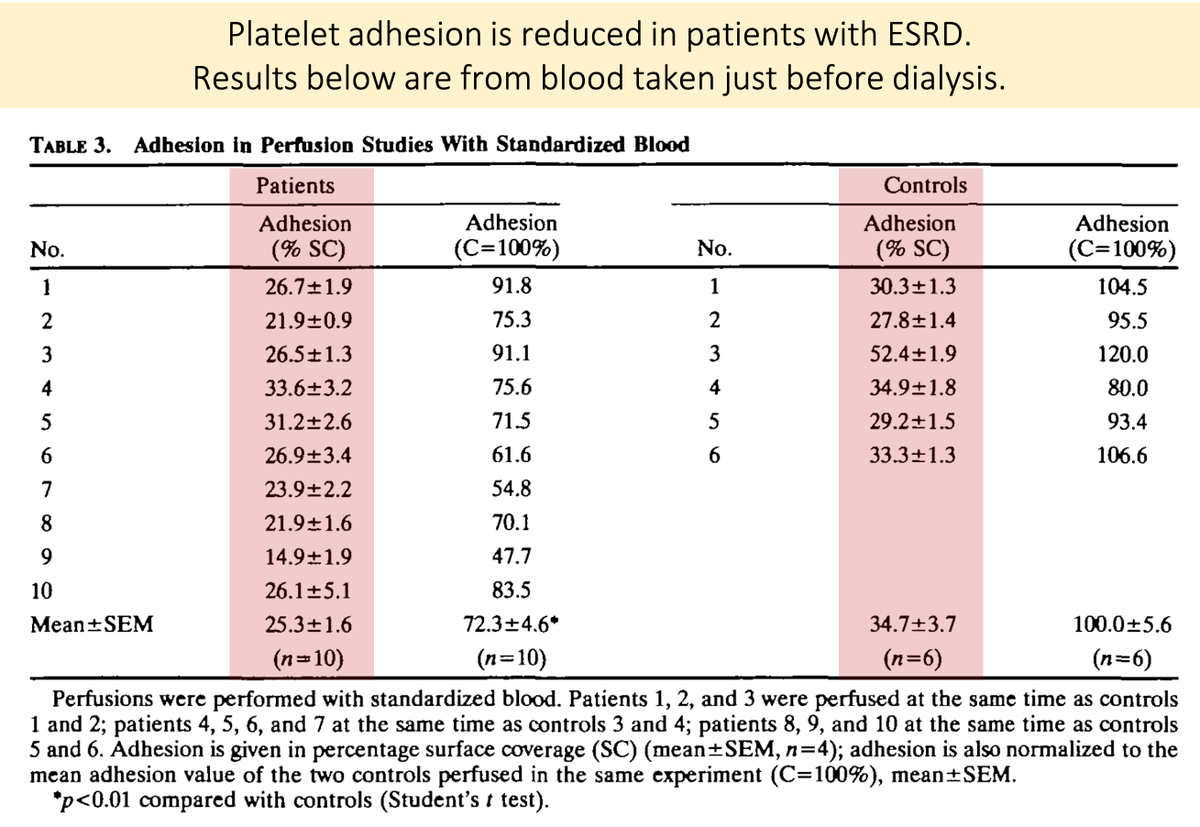
One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.
When normal platelets were placed in uremic plasma, the results were similar.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔑" title="Schlüssel" aria-label="Emoji: Schlüssel">Something in the plasma causes the problem!
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔑" title="Schlüssel" aria-label="Emoji: Schlüssel">Something in the plasma causes the problem!
https://pubmed.ncbi.nlm.nih.gov/2029508/ ">https://pubmed.ncbi.nlm.nih.gov/2029508/&...
One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.
When normal platelets were placed in uremic plasma, the results were similar.
https://pubmed.ncbi.nlm.nih.gov/2029508/ ">https://pubmed.ncbi.nlm.nih.gov/2029508/&...
4/
There are many potential "uremic toxins".
Which do you suspect is the culprit causing bleeding problems in patients with renal failure?
There are many potential "uremic toxins".
Which do you suspect is the culprit causing bleeding problems in patients with renal failure?
5/
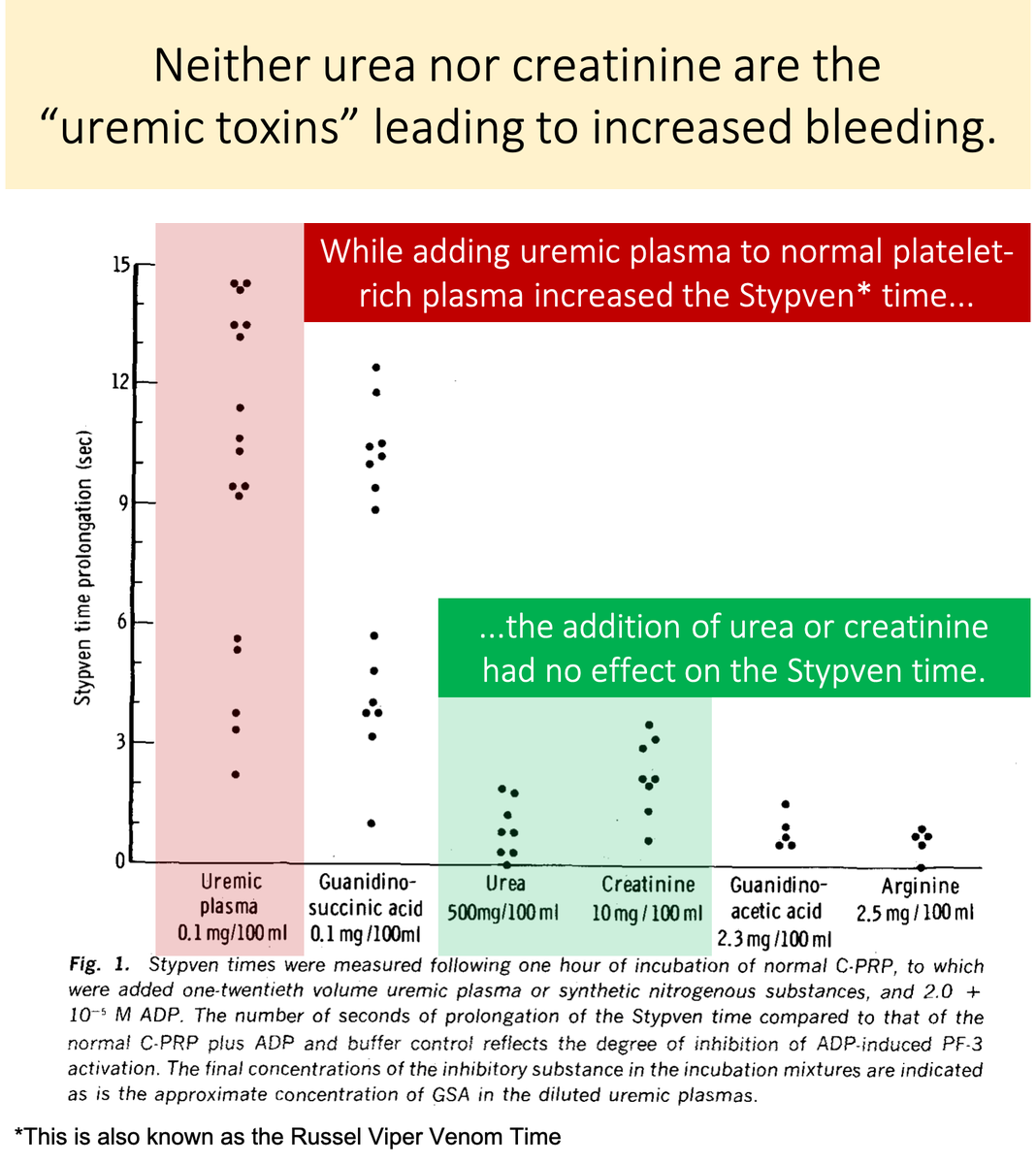
Neither urea nor creatinine cause uremic platelets.
In one study their addition to normal platelet-rich plasma had no effect on platelet function.
The toxin is something other than urea or creatinine!
https://pubmed.ncbi.nlm.nih.gov/5455565/ ">https://pubmed.ncbi.nlm.nih.gov/5455565/&...
Neither urea nor creatinine cause uremic platelets.
In one study their addition to normal platelet-rich plasma had no effect on platelet function.
The toxin is something other than urea or creatinine!
https://pubmed.ncbi.nlm.nih.gov/5455565/ ">https://pubmed.ncbi.nlm.nih.gov/5455565/&...
6/
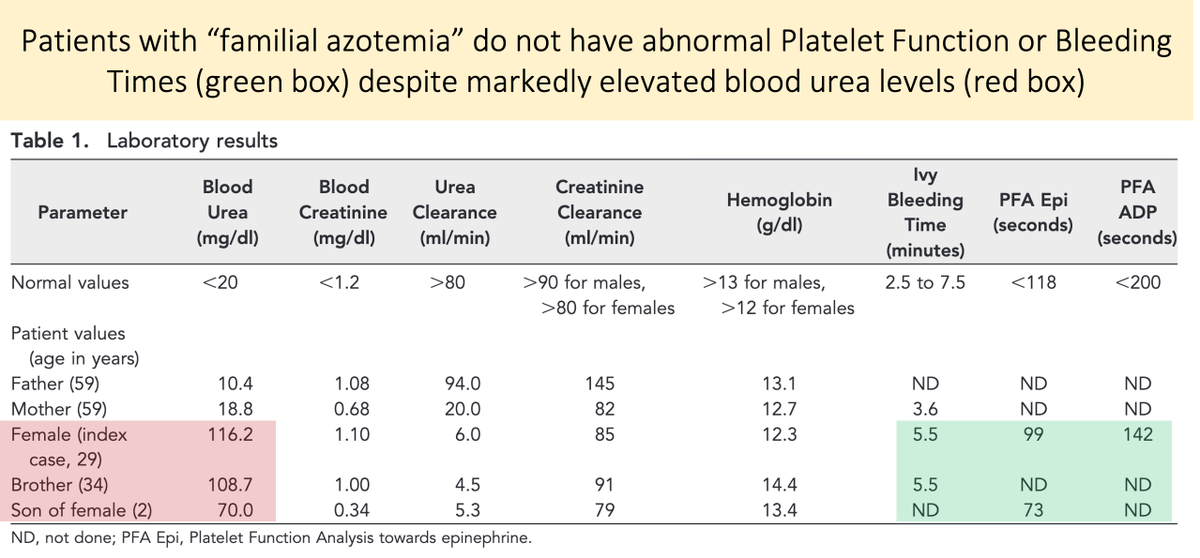
The lack of a role of urea as a uremic toxin is supported by the study of familial azotemia (a syndrome characterized by high plasma urea but normal renal function).
These patients do not experience uremic symptoms and have normal platelet function.
https://www.ncbi.nlm.nih.gov/pubmed/20360312 ">https://www.ncbi.nlm.nih.gov/pubmed/20...
The lack of a role of urea as a uremic toxin is supported by the study of familial azotemia (a syndrome characterized by high plasma urea but normal renal function).
These patients do not experience uremic symptoms and have normal platelet function.
https://www.ncbi.nlm.nih.gov/pubmed/20360312 ">https://www.ncbi.nlm.nih.gov/pubmed/20...
7/
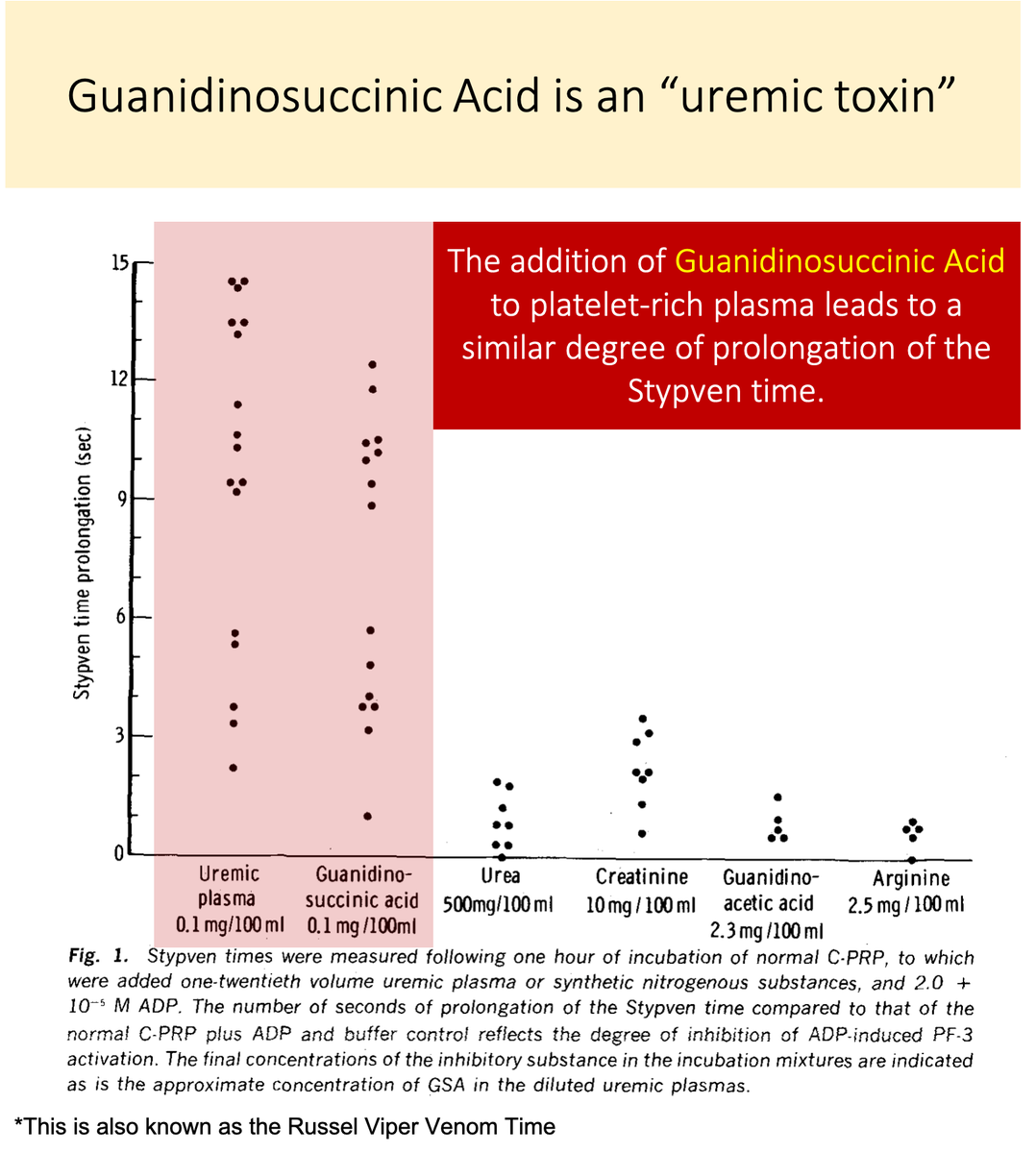
Let& #39;s look again at the study in tweet 5.
You& #39;ll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">Guanidinosuccinic acid
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">Guanidinosuccinic acid https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">
https://pubmed.ncbi.nlm.nih.gov/5455565/ ">https://pubmed.ncbi.nlm.nih.gov/5455565/&...
Let& #39;s look again at the study in tweet 5.
You& #39;ll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.
https://pubmed.ncbi.nlm.nih.gov/5455565/ ">https://pubmed.ncbi.nlm.nih.gov/5455565/&...
8/
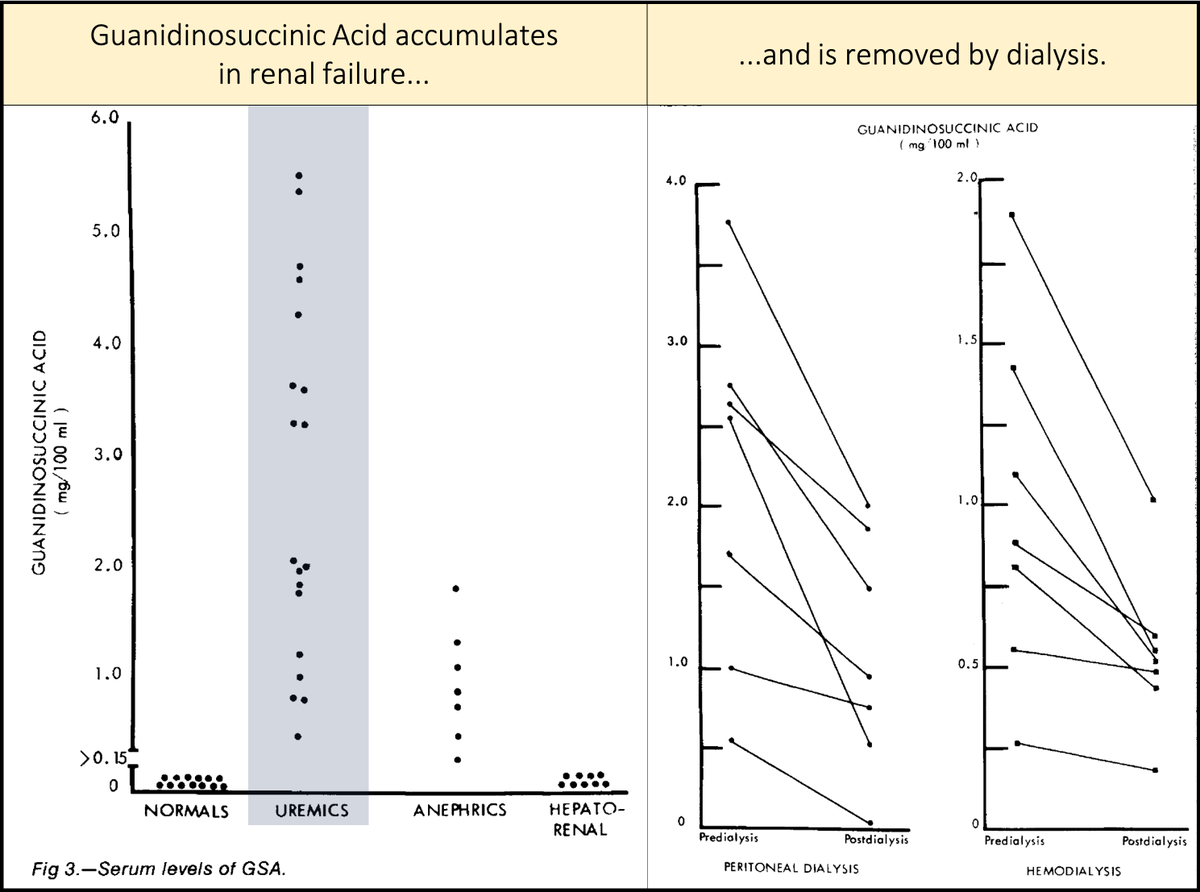
Key points about guanidinosuccinic acid (GSA):
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">It accumulates in renal failure
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">It accumulates in renal failure
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis
[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.]
https://pubmed.ncbi.nlm.nih.gov/5475708/ ">https://pubmed.ncbi.nlm.nih.gov/5475708/&...
Key points about guanidinosuccinic acid (GSA):
[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.]
https://pubmed.ncbi.nlm.nih.gov/5475708/ ">https://pubmed.ncbi.nlm.nih.gov/5475708/&...
9/
We now have two questions:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="1⃣" title="Tastenkappe Ziffer 1" aria-label="Emoji: Tastenkappe Ziffer 1">Why does GSA accumulate?
https://abs.twimg.com/emoji/v2/... draggable="false" alt="1⃣" title="Tastenkappe Ziffer 1" aria-label="Emoji: Tastenkappe Ziffer 1">Why does GSA accumulate?
&
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="2⃣" title="Tastenkappe Ziffer 2" aria-label="Emoji: Tastenkappe Ziffer 2">What does it do to platelets?
https://abs.twimg.com/emoji/v2/... draggable="false" alt="2⃣" title="Tastenkappe Ziffer 2" aria-label="Emoji: Tastenkappe Ziffer 2">What does it do to platelets?
We now have two questions:
&
10/
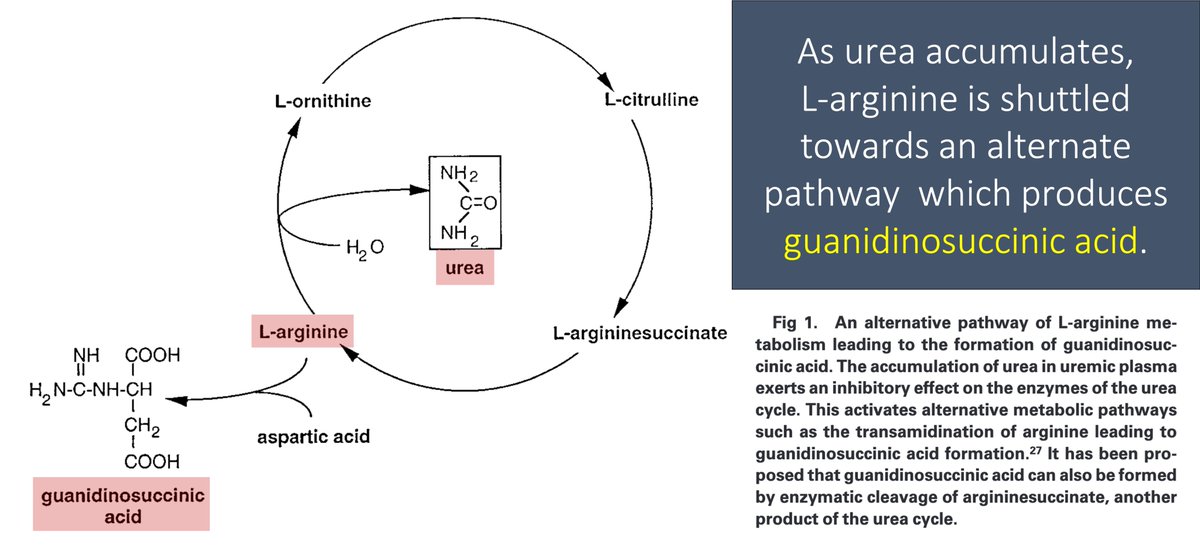
In renal failure, urea accumulates in the blood. As a result, the enzymes that convert ammonia to urea (the urea cycle!) are down-regulated.
As an alternative pathway to deal with toxic ammonia, L-arginine is converted to GSA.
GSA levels rise.
https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
In renal failure, urea accumulates in the blood. As a result, the enzymes that convert ammonia to urea (the urea cycle!) are down-regulated.
As an alternative pathway to deal with toxic ammonia, L-arginine is converted to GSA.
GSA levels rise.
https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
11/
To understand why GSA leads to platelet dysfunction, we must briefly review how nitric oxide (NO) affects hemostasis.
In addition to causing vasodilation (which helps promote blood flow and reduce hemostasis), NO also inhibits platelet function.
https://pubmed.ncbi.nlm.nih.gov/17901370/ ">https://pubmed.ncbi.nlm.nih.gov/17901370/...
To understand why GSA leads to platelet dysfunction, we must briefly review how nitric oxide (NO) affects hemostasis.
In addition to causing vasodilation (which helps promote blood flow and reduce hemostasis), NO also inhibits platelet function.
https://pubmed.ncbi.nlm.nih.gov/17901370/ ">https://pubmed.ncbi.nlm.nih.gov/17901370/...
12/
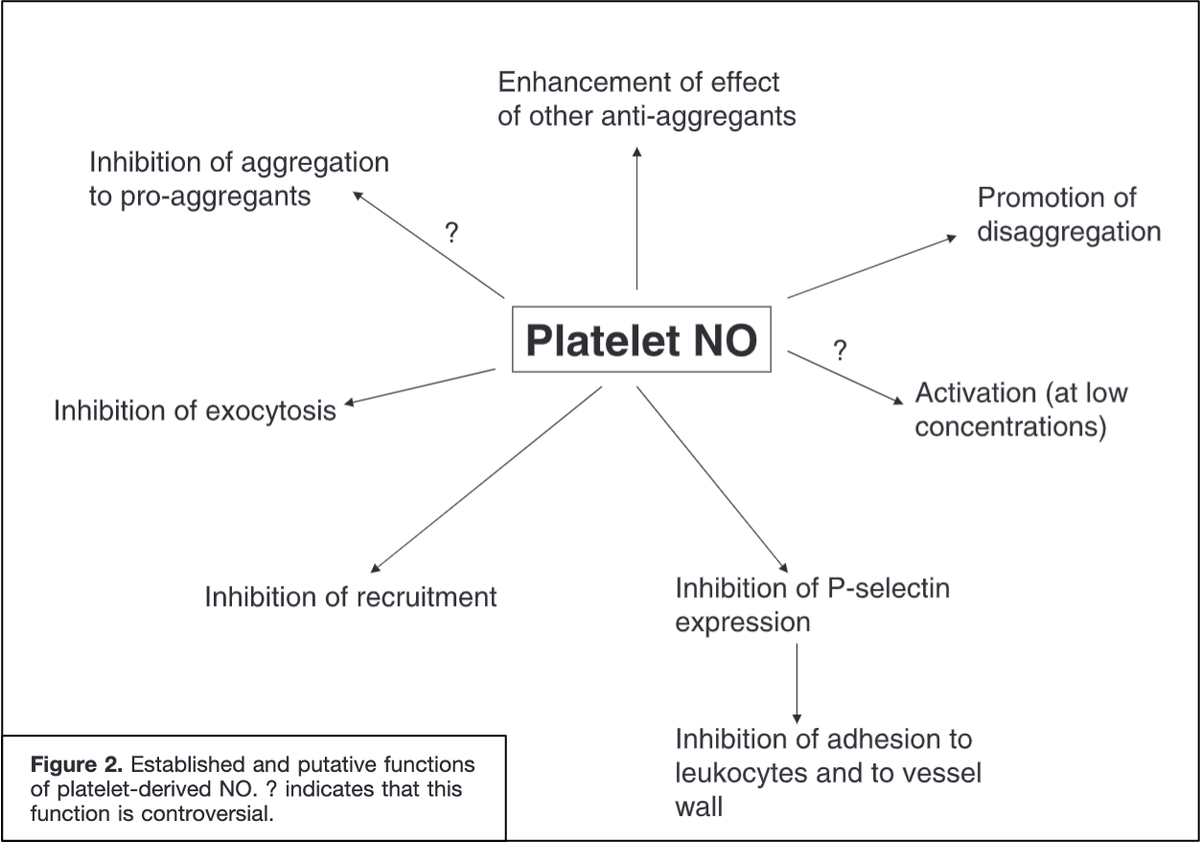
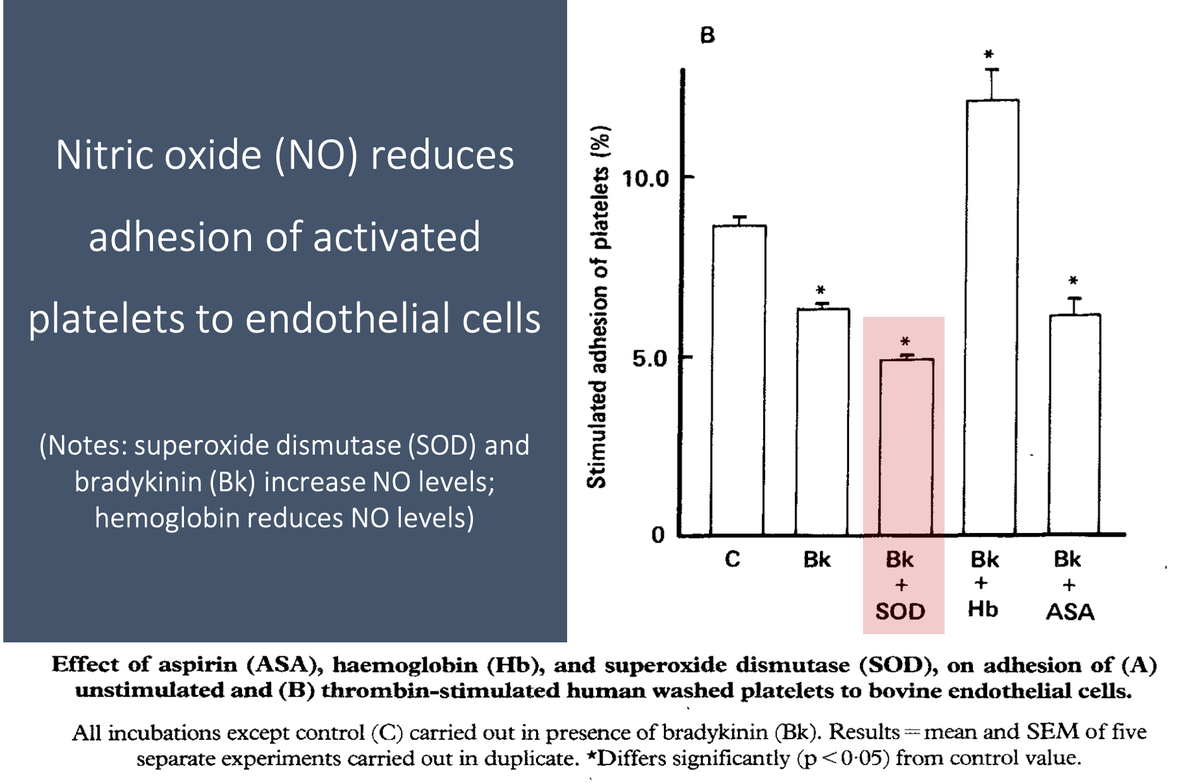
For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.
These effects are mediated by an increase in cyclic GMP (Link 2).
https://pubmed.ncbi.nlm.nih.gov/2889967/
https://pubmed.ncbi.nlm.nih.gov/2889967/&... href=" https://pubmed.ncbi.nlm.nih.gov/1695013/ ">https://pubmed.ncbi.nlm.nih.gov/1695013/&...
For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.
These effects are mediated by an increase in cyclic GMP (Link 2).
https://pubmed.ncbi.nlm.nih.gov/2889967/
13/
And how do these effects of nitric oxide relate to uremia and increased levels of GSA?
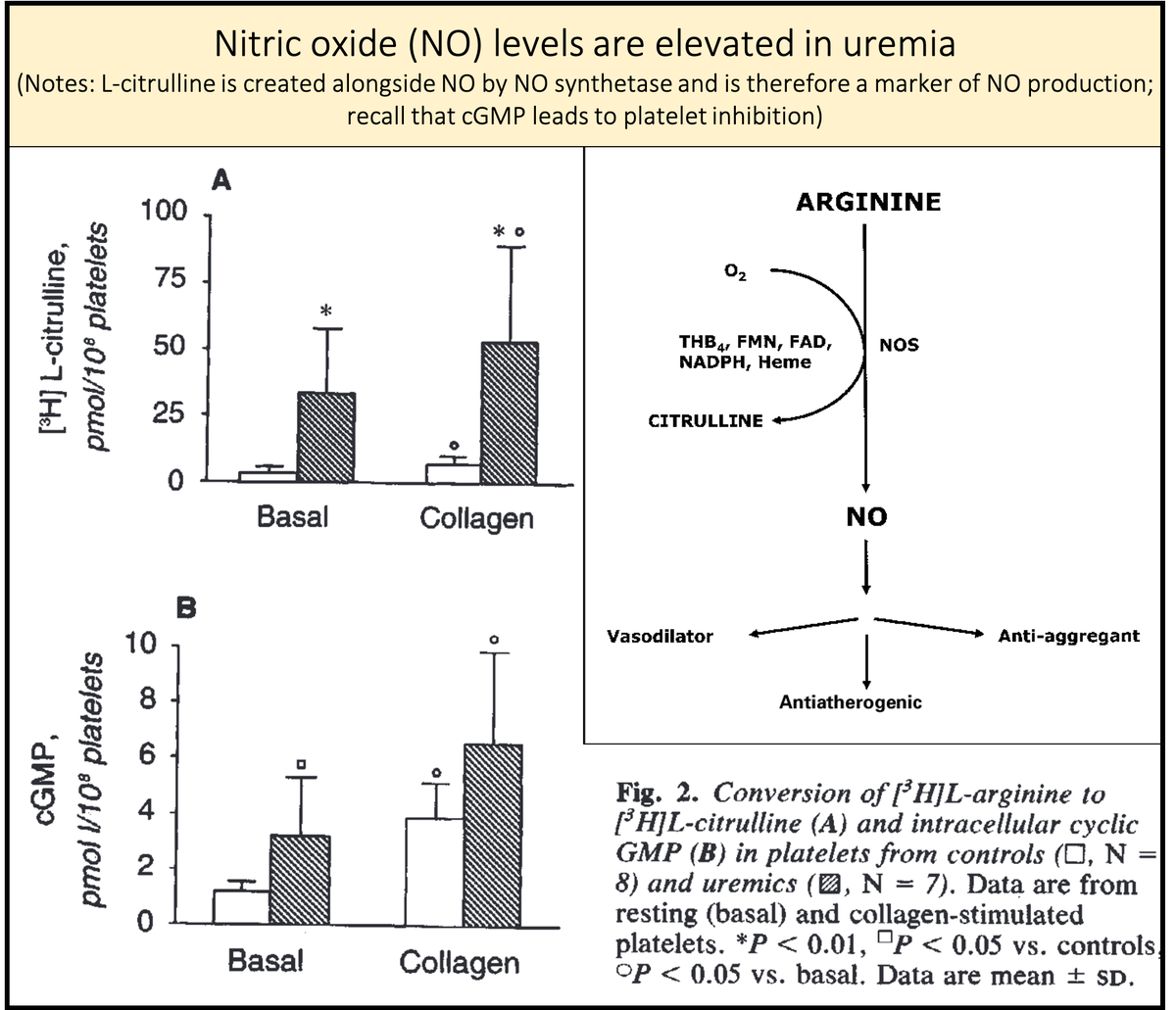
First, nitric oxide synthesis is enhanced in uremia. This may be the end-product leading to many of the features of uremic platelets.
https://pubmed.ncbi.nlm.nih.gov/8377388/ ">https://pubmed.ncbi.nlm.nih.gov/8377388/&...
And how do these effects of nitric oxide relate to uremia and increased levels of GSA?
First, nitric oxide synthesis is enhanced in uremia. This may be the end-product leading to many of the features of uremic platelets.
https://pubmed.ncbi.nlm.nih.gov/8377388/ ">https://pubmed.ncbi.nlm.nih.gov/8377388/&...
14/
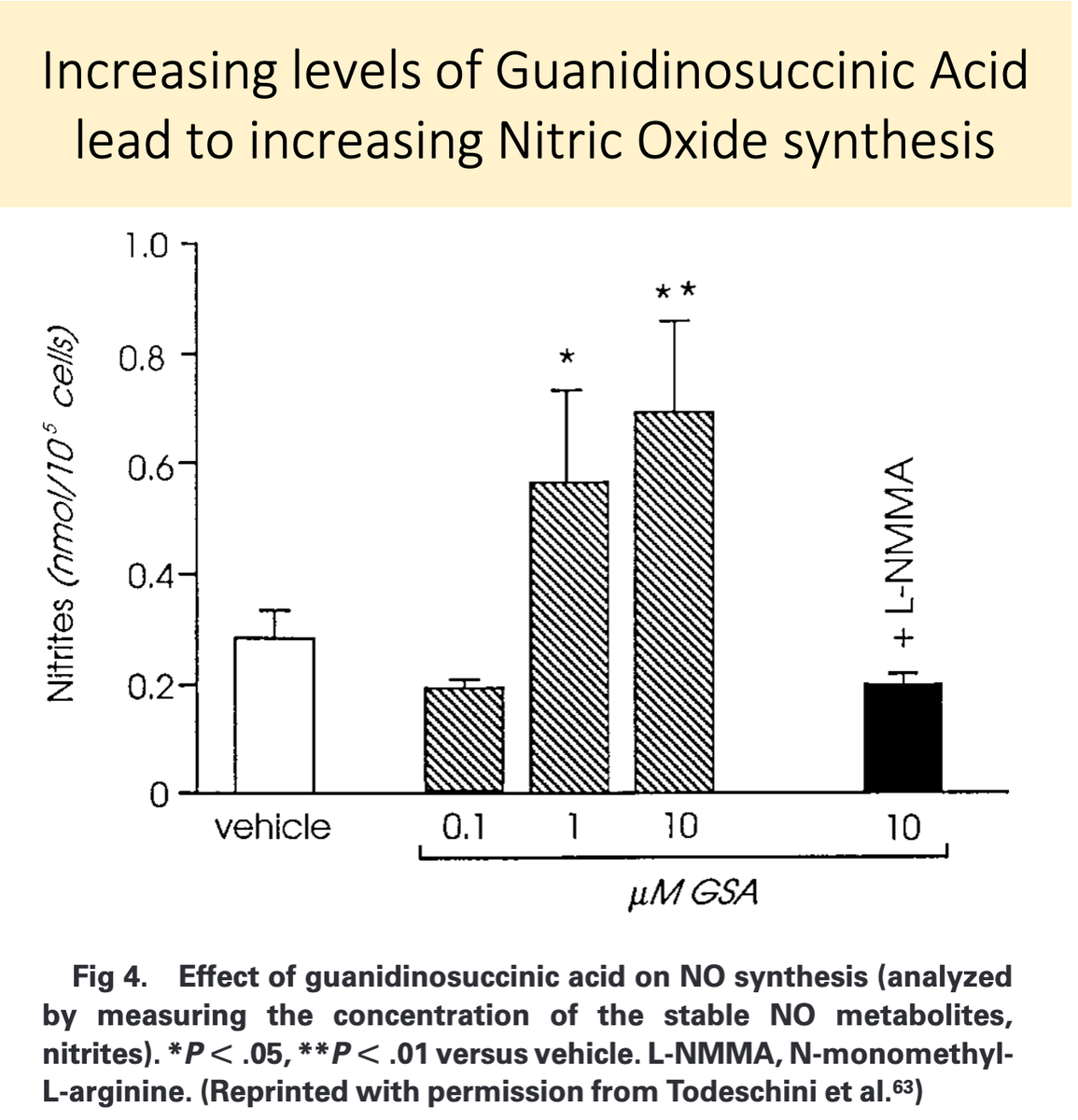
Second, because GSA is structurally similar to L-arginine, it is likely converted to nitric oxide in uremia.
GSA accumulates in renal failure and is converted to nitric oxide, leading to "uremic platelets"!
https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
Second, because GSA is structurally similar to L-arginine, it is likely converted to nitric oxide in uremia.
GSA accumulates in renal failure and is converted to nitric oxide, leading to "uremic platelets"!
https://pubmed.ncbi.nlm.nih.gov/10515859/ ">https://pubmed.ncbi.nlm.nih.gov/10515859/...
15/
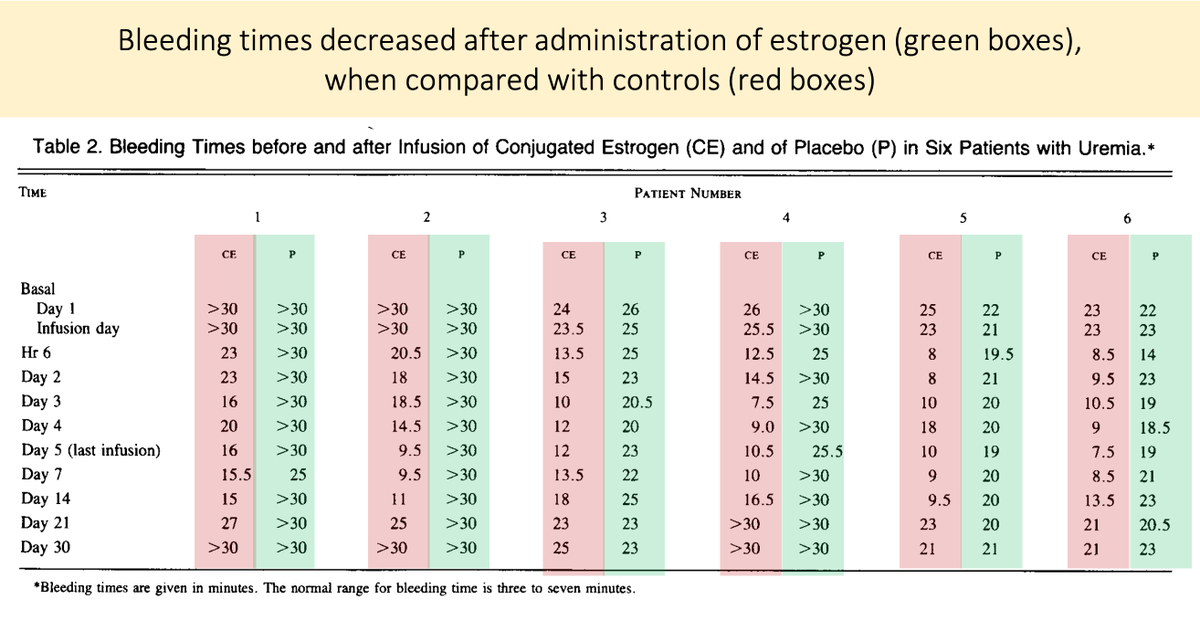
Before closing, there is an interesting clinical correlate.
Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).
The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2).
https://pubmed.ncbi.nlm.nih.gov/3018561/
https://pubmed.ncbi.nlm.nih.gov/3018561/&... href=" https://pubmed.ncbi.nlm.nih.gov/1656142/ ">https://pubmed.ncbi.nlm.nih.gov/1656142/&...
Before closing, there is an interesting clinical correlate.
Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).
The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2).
https://pubmed.ncbi.nlm.nih.gov/3018561/
16/16
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">Guanidinosuccinic acid (GSA) accumulates in renal failure
https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">Guanidinosuccinic acid (GSA) accumulates in renal failure
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">Increased GSA leads to increased nitric oxide (NO)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">Increased GSA leads to increased nitric oxide (NO)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">NO has multiple antiplatelet effects
https://abs.twimg.com/emoji/v2/... draggable="false" alt="◼️" title="Schwarzes durchschnittliches Quadrat" aria-label="Emoji: Schwarzes durchschnittliches Quadrat">NO has multiple antiplatelet effects
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">GSA is a uremic toxic that leads to uremic platelets
https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">GSA is a uremic toxic that leads to uremic platelets
[Reminder: there are MANY other mechanisms; see tweet 2.]
[Reminder: there are MANY other mechanisms; see tweet 2.]
I want to extend a HUGE thank you to...
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎗️" title="Erinnerungsband" aria-label="Emoji: Erinnerungsband">Justine Ryu ( @justine_ryu), Heme/Onc Fellow, Division of Hematology & Oncology, @BIDMChealth
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎗️" title="Erinnerungsband" aria-label="Emoji: Erinnerungsband">Justine Ryu ( @justine_ryu), Heme/Onc Fellow, Division of Hematology & Oncology, @BIDMChealth
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎗️" title="Erinnerungsband" aria-label="Emoji: Erinnerungsband">Jason Freed ( @FreedoBaggins), Hematologist, Division of Hematology & Oncology, @BIDMChealth
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🎗️" title="Erinnerungsband" aria-label="Emoji: Erinnerungsband">Jason Freed ( @FreedoBaggins), Hematologist, Division of Hematology & Oncology, @BIDMChealth
...for their peer review of this tweetorial!
...for their peer review of this tweetorial!
Very appropriate question. From what I understand, there has been a lot of discussion about the appropriateness of VKA and DOACs in ESRD.
As with anything, it is a risk/benefit issue.
Here is a recent study on warfarin.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2763969">https://jamanetwork.com/journals/... https://twitter.com/jwgroenendyk/status/1283487427345240065?s=20">https://twitter.com/jwgroenen...
As with anything, it is a risk/benefit issue.
Here is a recent study on warfarin.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2763969">https://jamanetwork.com/journals/... https://twitter.com/jwgroenendyk/status/1283487427345240065?s=20">https://twitter.com/jwgroenen...
This is a great thought, Avi. I simplistically viewed DDAVP as increasing VFW, but this connection with nitric oxide is intriguing! https://twitter.com/AvrahamCooperMD/status/1283493430748995585?s=20">https://twitter.com/AvrahamCo...
Another great clinical correlate. Thank you adding, Jason.
https://twitter.com/freedobaggins/status/1283495577418788864?s=21">https://twitter.com/freedobag... https://twitter.com/freedobaggins/status/1283495577418788864">https://twitter.com/freedobag...
https://twitter.com/freedobaggins/status/1283495577418788864?s=21">https://twitter.com/freedobag... https://twitter.com/freedobaggins/status/1283495577418788864">https://twitter.com/freedobag...
Absolutely fascinating!
https://twitter.com/freedobaggins/status/1283500832051261440?s=21">https://twitter.com/freedobag... https://twitter.com/freedobaggins/status/1283500832051261440">https://twitter.com/freedobag...
https://twitter.com/freedobaggins/status/1283500832051261440?s=21">https://twitter.com/freedobag... https://twitter.com/freedobaggins/status/1283500832051261440">https://twitter.com/freedobag...

 Read on Twitter
Read on Twitter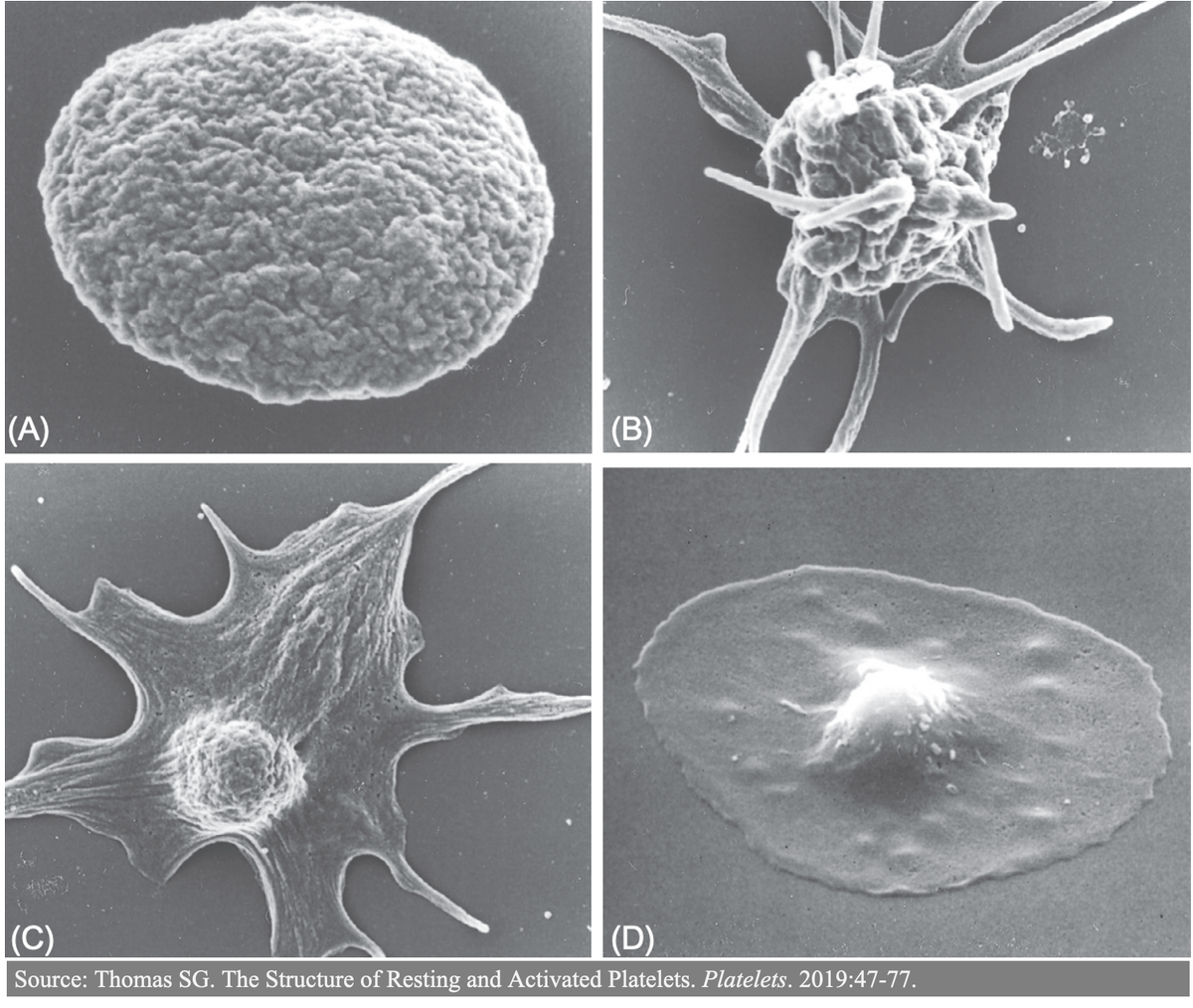
 Something in the plasma causes the problem! https://pubmed.ncbi.nlm.nih.gov/2029508/&..." title="3/One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.When normal platelets were placed in uremic plasma, the results were similar.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔑" title="Schlüssel" aria-label="Emoji: Schlüssel">Something in the plasma causes the problem! https://pubmed.ncbi.nlm.nih.gov/2029508/&..." class="img-responsive" style="max-width:100%;"/>
Something in the plasma causes the problem! https://pubmed.ncbi.nlm.nih.gov/2029508/&..." title="3/One study took blood from patients with ESRD just before dialysis and found reductions in platelet adhesion and aggregation.When normal platelets were placed in uremic plasma, the results were similar.https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔑" title="Schlüssel" aria-label="Emoji: Schlüssel">Something in the plasma causes the problem! https://pubmed.ncbi.nlm.nih.gov/2029508/&..." class="img-responsive" style="max-width:100%;"/>
 Guanidinosuccinic acidhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> https://pubmed.ncbi.nlm.nih.gov/5455565/&..." title="7/Let& #39;s look again at the study in tweet 5.You& #39;ll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">Guanidinosuccinic acidhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> https://pubmed.ncbi.nlm.nih.gov/5455565/&..." class="img-responsive" style="max-width:100%;"/>
Guanidinosuccinic acidhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> https://pubmed.ncbi.nlm.nih.gov/5455565/&..." title="7/Let& #39;s look again at the study in tweet 5.You& #39;ll notice that there IS a substance that, when added to normal platelet-rich plasma, negatively affects platelet function.https://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision">Guanidinosuccinic acidhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="💥" title="Symbol für eine Kollision" aria-label="Emoji: Symbol für eine Kollision"> https://pubmed.ncbi.nlm.nih.gov/5455565/&..." class="img-responsive" style="max-width:100%;"/>
 It accumulates in renal failurehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.] https://pubmed.ncbi.nlm.nih.gov/5475708/&..." title="8/Key points about guanidinosuccinic acid (GSA):https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">It accumulates in renal failurehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.] https://pubmed.ncbi.nlm.nih.gov/5475708/&..." class="img-responsive" style="max-width:100%;"/>
It accumulates in renal failurehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.] https://pubmed.ncbi.nlm.nih.gov/5475708/&..." title="8/Key points about guanidinosuccinic acid (GSA):https://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">It accumulates in renal failurehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▪️" title="Schwarzes kleines Quadrat" aria-label="Emoji: Schwarzes kleines Quadrat">Is removed by dialysis[Note: though not all studies agree, dialysis is generally felt to mitigate platelet dysfunction by removal of a toxin.] https://pubmed.ncbi.nlm.nih.gov/5475708/&..." class="img-responsive" style="max-width:100%;"/>
 https://pubmed.ncbi.nlm.nih.gov/1695013/&..." title="12/For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.These effects are mediated by an increase in cyclic GMP (Link 2). https://pubmed.ncbi.nlm.nih.gov/2889967/&... href=" https://pubmed.ncbi.nlm.nih.gov/1695013/ ">https://pubmed.ncbi.nlm.nih.gov/1695013/&..." class="img-responsive" style="max-width:100%;"/>
https://pubmed.ncbi.nlm.nih.gov/1695013/&..." title="12/For example, nitric oxide reduces platelet adhesion to the endothelium (Link 1 and Picture) and platelet-platelet aggregation.These effects are mediated by an increase in cyclic GMP (Link 2). https://pubmed.ncbi.nlm.nih.gov/2889967/&... href=" https://pubmed.ncbi.nlm.nih.gov/1695013/ ">https://pubmed.ncbi.nlm.nih.gov/1695013/&..." class="img-responsive" style="max-width:100%;"/>
 https://pubmed.ncbi.nlm.nih.gov/1656142/&..." title="15/Before closing, there is an interesting clinical correlate.Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2). https://pubmed.ncbi.nlm.nih.gov/3018561/&... href=" https://pubmed.ncbi.nlm.nih.gov/1656142/ ">https://pubmed.ncbi.nlm.nih.gov/1656142/&..." class="img-responsive" style="max-width:100%;"/>
https://pubmed.ncbi.nlm.nih.gov/1656142/&..." title="15/Before closing, there is an interesting clinical correlate.Estrogens are an effective treatment for uremic bleeding (Link 1 and Picture).The proposed mechanism? Estrogen-mediated reduction in nitric oxide! (Link 2). https://pubmed.ncbi.nlm.nih.gov/3018561/&... href=" https://pubmed.ncbi.nlm.nih.gov/1656142/ ">https://pubmed.ncbi.nlm.nih.gov/1656142/&..." class="img-responsive" style="max-width:100%;"/>


